추천 제품
형태
solid
색상
off-white
solubility
DMF: soluble
저장 온도
2-8°C
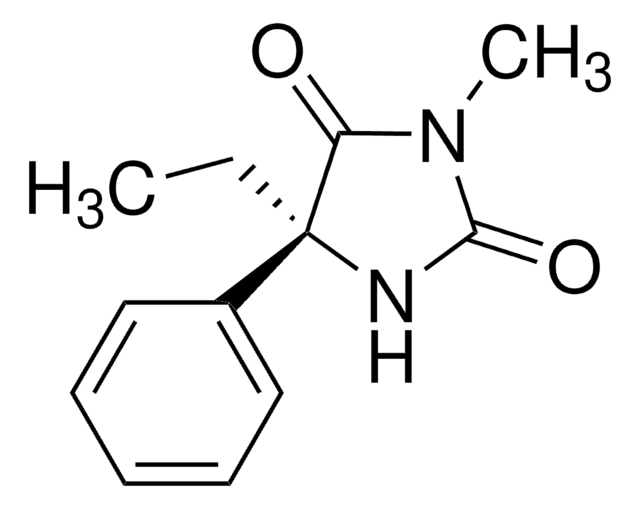
SMILES string
CC[C@@]1(NC(=O)NC1=O)c2ccccc2
InChI
1S/C11H12N2O2/c1-2-11(8-6-4-3-5-7-8)9(14)12-10(15)13-11/h3-7H,2H2,1H3,(H2,12,13,14,15)/t11-/m1/s1
InChI key
UDTWZFJEMMUFLC-LLVKDONJSA-N
생화학적/생리학적 작용
CYP2B6 metabolite of (S)-(+)-mephenytoin; anticonvulsant; hypnotic.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
제조 메모
Nirvanol is soluble in DMF.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
W Kalow
Xenobiotica; the fate of foreign compounds in biological systems, 16(5), 379-389 (1986-05-01)
The antiepileptic drug mephenytoin is a racemate. Mephenytoin hydroxylation is a stereospecific reaction and is confined to the S-enantiomer, which is normally eliminated within hours, allowing the R-enantiomer to accumulate since it can be eliminated only within days or weeks.
W H Theodore et al.
Neurology, 34(8), 1100-1102 (1984-08-01)
We investigated the conversion of mephenytoin to nirvanol in five patients with uncontrolled complex partial seizures. After a 50-mg single oral dose, mean peak mephenytoin level was 0.48 microgram/ml and nirvanol 0.37 microgram/ml. After 400 mg, peak mephenytoin level was
S H Akrawi et al.
European journal of drug metabolism and pharmacokinetics, 14(4), 269-278 (1989-10-01)
The stereoselective clearances of R- and S-mephenytoin were determined in rats receiving either an intravenous or hepatic portal vein infusion of racemic mephenytoin. The mean +/- SD intravenous clearances of R- and S-mephenytoin were 1630 +/- 250 ml/hr and 630
A Pezeshk et al.
Journal of inorganic biochemistry, 42(4), 267-272 (1991-06-01)
The preparation and spectral properties of copper(II) complexes of two hydantoins are reported. Complexes of the general formula Cu(hyd)2(py)2, where hyd = phenytoin or nirvanol; and py = pyridine were prepared and characterized by infrared and ESR. Spectral data show
B F Bourgeois et al.
Epilepsia, 27(4), 412-418 (1986-07-01)
Stereoselective metabolism has been demonstrated for mephenytoin (MHT), R-MHT being demethylated to the pharmacologically active metabolite 5-phenyl-5-ethylhydantoin (PEH; nirvanol), and S-MHT undergoing aromatic hydroxylation to 4-OH-MHT, with formation of an intermediate arene oxide metabolite. PEH is responsible for the therapeutic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.