77440
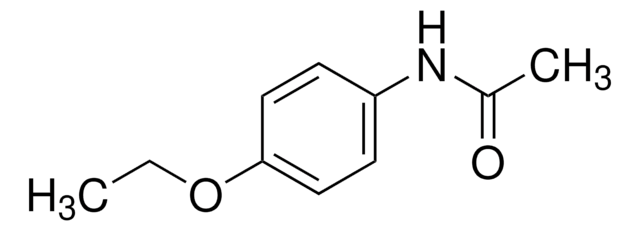
Phenacetin
≥98.0% (HPLC)
동의어(들):
1-Acetyl-p-phenetidin, 4′-Ethoxyacetanilide, N-(4-Ethoxyphenyl)acetamide, p-Acetophenetidide, Acetophenetidin
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
Linear Formula:
CH3CONHC6H4OC2H5
CAS Number:
Molecular Weight:
179.22
Beilstein:
1869238
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Phenacetin (phen) can be used to synthesize the charge-transfer (CT) complex [(phen)(TCNE)12] by reacting with tetracyanoethylene (TCNE) in dichloromethane.
생화학적/생리학적 작용
Substrate of CYP1A2 and CYP2D6.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Spectroscopic investigation of the novel charge-transfer complex [(phen)(TCNE)12] formed in the reaction of phenacetin with tetracyanoethylene.
AlQaradawi SY and Nour EM
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 62(1-3), 578-581 (2005)
J A Hinson
Environmental health perspectives, 49, 71-79 (1983-03-01)
Phenacetin can be metabolized to reactive metabolites by a variety of mechanisms. (1) Phenacetin can be N-hydroxylated, and the resulting N-hydroxyphenacetin can be sulfated or glucuronidated. Whereas phenacetin N-O sulfate immediately rearranges to form a reactive metabolite which may covalently
S P Clissold
Drugs, 32 Suppl 4, 46-59 (1986-01-01)
Since their synthesis in the late 1800s paracetamol (acetaminophen) and phenacetin have followed divergent pathways with regard to their popularity as mild analgesic/antipyretic drugs. Initially, paracetamol was discarded in favour of phenacetin because the latter drug was supposedly less toxic.
[Phenacetin abuse I. Occurrence, per capita consumption and costs of treatment].
M J Mihatsch et al.
Schweizerische medizinische Wochenschrift, 110(4), 108-115 (1980-01-28)
Heloisa N Bordallo et al.
Molecular pharmaceutics, 9(9), 2434-2441 (2012-07-25)
This study centers on the use of inelastic neutron scattering as an alternative tool for physical characterization of solid pharmaceutical drugs. On the basis of such approach, relaxation processes in the pharmaceutical compound phenacetin (p-ethoxyacetanilide, C(10)H(13)NO(2)) were evidenced on heating
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.