SML3737
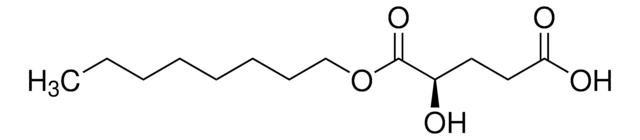
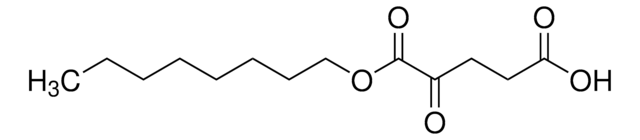
Octyl-(S)-2HG
≥98% (HPLC)
동의어(들):
(2S)-2-Hydroxyglutarate octyl ester, (2S)-Octyl-α-hydroxyglutarate, (S)-4-Hydroxy-5-(octyloxy)-5-oxopentanoic acid, 1-Octyl-L-2-hydroxyglutarate, 2S-Hydroxy-pentanedioic acid, 1-octyl ester, L-Octyl-2HG, L2HG, Octyl-(S)-2-hydroxyglutarate, Octyl-L-2HG, S-2HG octyl ester
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C13H24O5
CAS Number:
Molecular Weight:
260.33
MDL number:
UNSPSC 코드:
12352200
NACRES:
NA.21
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: 2 mg/mL, clear
저장 온도
-10 to -25°C
SMILES string
O[C@@H](CCC(=O)O)C(=O)OCCCCCCCC
InChI
1S/C13H24O5/c1-2-3-4-5-6-7-10-18-13(17)11(14)8-9-12(15)16/h11,14H,2-10H2,1H3,(H,15,16)/t11-/m0/s1
InChI key
UJZOKTKSGUOCCM-NSHDSACASA-N
생화학적/생리학적 작용
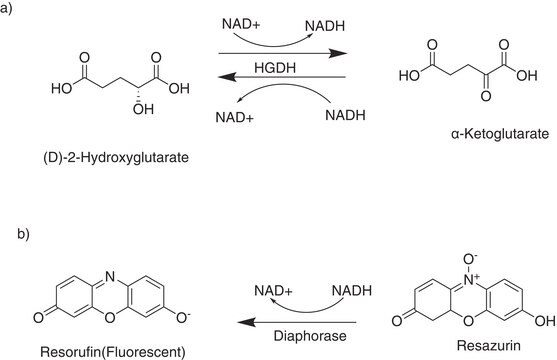
Membrane-permeant precursor form of the oncometabolite L-2-hydroxyglutarate that inhibits α-ketoglutarate/α-KG-dependent dioxygenases.
Octyl-(S)-2HG (Octyl-L-2HG) is a membrane-permeant precursor form of the oncometabolite L-2-hydroxyglutarate (L2HG) produced from α‐KG by malate dehydrogenases (MDH1/2) and lactate dehydrogenase (LDHA). L2HG can be converted back to α-KG by L-2-hydroxyglutarate dehydrogenase (LHGDH), deletion or mutations of which lead to L2HG accumulation, metabolic disorders and reduced 5hmC levels. Both D- and L-2HG inhibit Jumonji histone demethylases and Tet oxygenases by competing against α-KG binding. However, L2HG is an antagonist, while R2HG (D2HG) is an agonist of α-KG-dependent prolylhydroxylase (EglN).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.