추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to off-white
solubility
DMSO: >25 mg/mL
저장 온도
room temp
SMILES string
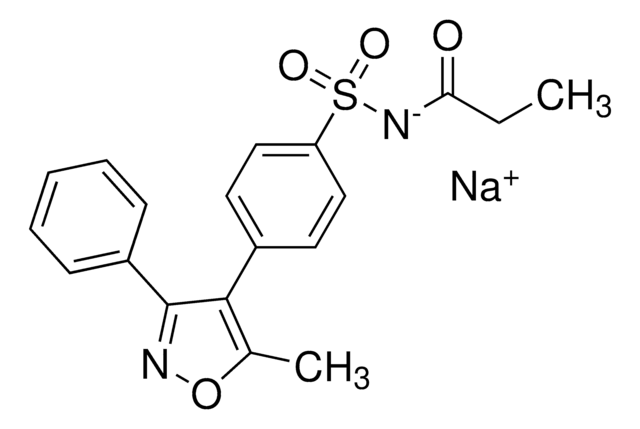
Cc1onc(-c2ccccc2)c1-c3ccc(cc3)S(N)(=O)=O
InChI
1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20)
InChI key
LNPDTQAFDNKSHK-UHFFFAOYSA-N
유전자 정보
human ... PTGS2(5743)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Valdecoxib (VCX) is a diaryl substituted isoxazole compound. It comprises of sulfonyl propanamide and is a metabolite of parecoxib.
애플리케이션
Valdecoxib may be used: as cyclooxygenase-2 (COX-2) inhibitor in fibroblast cells, as an analyte for mass spectrometry analysis, as an standard in ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) for quantification of valdecoxib in plasma samples
생화학적/생리학적 작용
Valdecoxib is a non-steroidal anti-inflammatory drug (NSAID), a cyclooxygenase-2 (COX-2) selective inhibitor.
Valdecoxib is reported to elicit anti-inflammatory, analgesic and antipyretic functionality. It acts as a substrate for the liver enzyme cytochrome P450 2C9(CYP2C9) and cytochrome P450 3A4 (CYP3A4).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 1 - Repr. 2 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Shuang-Long Li et al.
Drug design, development and therapy, 14, 1117-1125 (2020-03-28)
A method for the simultaneous determination of parecoxib and its metabolite valdecoxib in beagle plasma by UPLC-MS/MS was developed and validated. After the plasma was extracted by acetonitrile precipitation, the analytes were separated on an Acquity UPLC BEH C18 column
Mari-Pau Mena et al.
Experimental cell research, 324(2), 124-136 (2014-03-25)
The mechanisms controlling the switch between the pro-angiogenic and pro-inflammatory states of endothelial cells are still poorly understood. In this paper, we show that: (a) COX-2 expression induced by VEGF-A is NFAT2-dependent; and (b) the integrin profile in endothelial cells
Guangbing Wei et al.
Drug design, development and therapy, 9, 3083-3098 (2015-06-26)
Postoperative intra-abdominal adhesions are common complications after abdominal surgery. The exact molecular mechanisms that are responsible for these complications remain unclear, and there are no effective methods for preventing adhesion formation or reformation. The aim of the study reported here
Melina Schellhorn et al.
Oncotarget, 6(36), 39342-39356 (2015-10-30)
The antitumorigenic mechanism of the selective cyclooxygenase-2 (COX-2) inhibitor celecoxib is still a matter of debate. Using lung cancer cell lines (A549, H460) and metastatic cells derived from a lung cancer patient, the present study investigates the impact of celecoxib
Sara Triñanes et al.
Journal of chromatography. A, 1420, 35-45 (2015-10-18)
The development and performance evaluation of a method for the simultaneous determination of six pharmaceuticals belonging to the class of non-steroidal anti-inflammatory drugs (NSAIDs) which present high selectivity for the cyclooxygenase (COX)-2 isoform of COX (COXIBs) in environmental waters are
관련 콘텐츠
Discover Bioactive Small Molecules for Lipid Signaling Research
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.