추천 제품
일반 설명
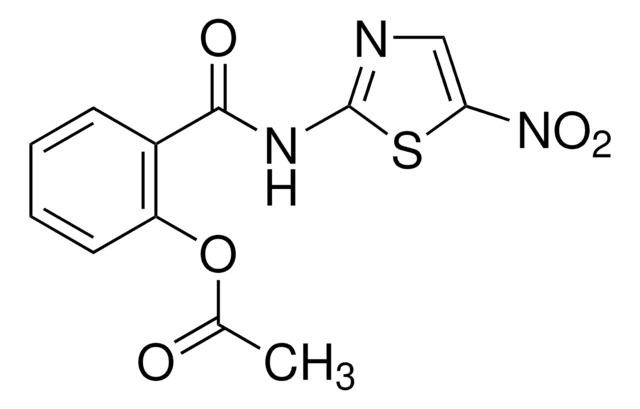
Nitazoxanide (NTZ), a thiazolide compound is a antiparasitic drug with structure similar to niclosamide.
애플리케이션
Nitazoxanide has been used:
- to test its anti-viral activity against chikungunya virus
- as an antiprotozoal agent to test its effect on cell viability in various cancer cell lines
- to test its effect on human cytomegalovirus (HCMV) infected human fibroblast HFF cells
생화학적/생리학적 작용
Nitazoxanide (NTZ) promotes autophagy by acting on kinase based signaling pathways and acts on mammalian target of rapamycin complex 1 (mTORC1) in Mycobacteria. It has anti-viral property and effectively halts entry and release of chikungunya virus in in vitro studies. NTZ also inhibits Japanese encephalitis virus (JEV) infection in early stages and has the potential to treat other viral infections including dengue, hepatitis B (HBV), coronavirus and human immunodeficiency virus (HIV). It has antineoplastic functionality and may induce apoptosis by promoting proto-oncogene c-Myc inhibition resulting in tumor suppression.
Nitazoxanide is an inhibitor of pyruvate-ferredoxin oxidoreductase (PFOR); FDA approved anti-parasitic drug (2002). Recent work (C & EN Sept. 14, 2009, p. 28) highlights that NTZ kills non-replicating and replicating TB bacteria and no apparent resistance is detected.
특징 및 장점
This compound was developed by Romark. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Benjamin Speich et al.
Parasites & vectors, 6, 3-3 (2013-01-08)
Pathogenic intestinal protozoa infections are common in school-aged children in the developing world and they are frequently associated with malabsorption syndromes and gastrointestinal morbidity. Since diagnosis of these parasites is difficult, prevalence data on intestinal protozoa is scarce. We collected
Jean-François Rossignol et al.
Transactions of the Royal Society of Tropical Medicine and Hygiene, 106(3), 167-173 (2012-02-04)
We conducted a double-blind, placebo-controlled clinical trial to demonstrate the efficacy of nitazoxanide suspension for the treatment of presumed infectious diarrhea in children. Eligible patients must have had diarrheal illness lasting 3-29 days. Patients were randomized to receive either nitazoxanide
Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis
Lam KYK
PLoS Pathogens, 8(5), 340-351 (2012)
Irit Krause et al.
The Pediatric infectious disease journal, 31(11), 1135-1138 (2012-07-20)
Cryptosporidium parvum is a common cause of diarrhea. In immunocompetent individuals, spontaneous recovery is the rule. In immunocompromised patients, it may cause a serious disease. Data on cryptosporidiosis in children after solid organ transplantation are few. We report on 6
Vanessa R Anderson et al.
Drugs, 67(13), 1947-1967 (2007-08-29)
Nitazoxanide (Alinia, Daxon, Dexidex, Paramix, Kidonax, Colufase, Annita) has in vitro activity against a variety of microorganisms, including a broad range of protozoa and helminths. Nitazoxanide is effective in the treatment of protozoal and helminthic infections, including Cryptosporidium parvum or
관련 콘텐츠
We offer agonists, antagonists, modulators and other bioactive small molecules for immune system signaling target identification and validation, as well as a variety of antibiotics, antivirals, and antifungals.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.