추천 제품
분석
≥98% (TLC)
양식
powder
solubility
H2O: insoluble
저장 온도
−20°C
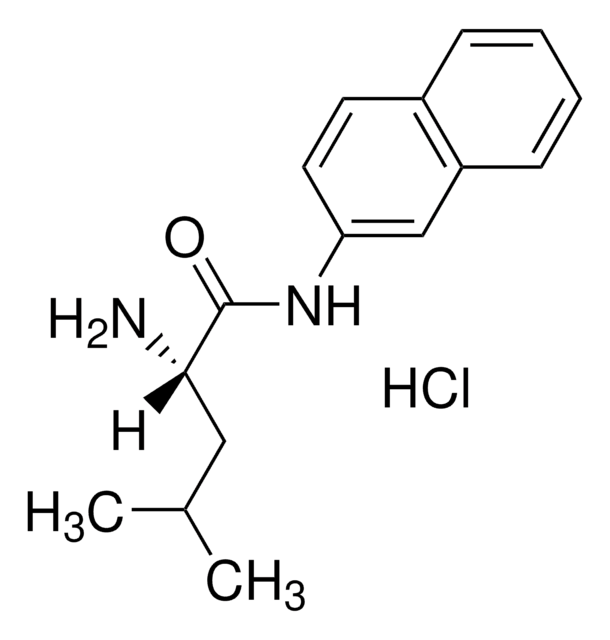
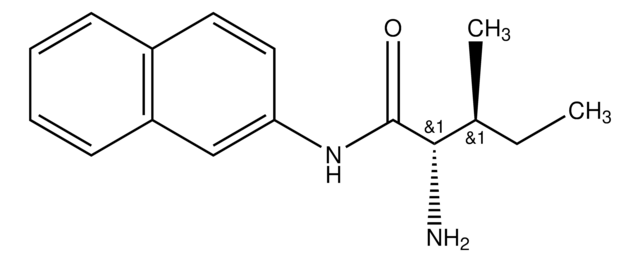
SMILES string
CC(C)C[C@H](N)C(=O)Nc1ccc2ccccc2c1
InChI
1S/C16H20N2O/c1-11(2)9-15(17)16(19)18-14-8-7-12-5-3-4-6-13(12)10-14/h3-8,10-11,15H,9,17H2,1-2H3,(H,18,19)/t15-/m0/s1
InChI key
JWHURRLUBVMKOT-HNNXBMFYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
L-Leucine β-naphthylamide has been used as a substrate:
- to measure the activity of aminopeptidase in Escherichia coli
- to evaluate the enzyme activity of cathepsin H from rabbit skeletal muscles
- in the proteolytic assay of L-Leucine aminopeptidase
Substrate for leucine aminopeptidase determination in colorimetric and histochemical procedures.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
품질
Very low free β-naphthylamine.
기질
Substrate for aminopeptidase M
이미 열람한 고객
Proteolytic activity in the placenta, decidua and postimplantation embryos of the rat.
A Fein et al.
Israel journal of medical sciences, 21(4), 394-396 (1985-04-01)
A M Reisenauer et al.
Science (New York, N.Y.), 227(4682), 70-72 (1985-01-04)
The regulation of amino-oligopeptidase (AOP), an intestinal brush border hydrolase essential for the surface digestion of peptide nutrients, was examined in rats in vivo. Short-term (30-minute) intraintestinal perfusion of a tetrapeptide substrate, Gly-Leu-Gly-Gly, or a synthetic substrate, leucyl-beta-naphthylamide, induced a
T Nishimura et al.
European journal of biochemistry, 137(1-2), 23-27 (1983-12-01)
The mode of action towards oligopeptides and proteins of hydrolase H purified from rabbit skeletal muscle was studied. The presence of protamine or alpha-N-benzoylarginine p-nitroanilide (an endopeptidase substrate) changed both the Km and V values of the enzyme towards Leu-beta-naphthylamide
Aminopeptidase (arylamidase) activity in discrete areas of the rat brain: sex differences.
J M de Gandarias et al.
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 21(5), 285-286 (1989-05-01)
Masanori Matsuishi et al.
The international journal of biochemistry & cell biology, 35(4), 474-485 (2003-02-05)
Rabbit muscle cathepsin H classified as an aminoendopeptidase was purified and its properties were investigated to clarify its contribution to the proteolysis of postmortem muscle. The purification was performed by ammonium sulfate fractionation and successive chromatographies on Sephadex G-75, phosphocelluose
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.