추천 제품
분석
≥98% (TLC)
Quality Level
양식
powder
solubility
1 M HCl: 49.00-51.00 mg/mL, clear to very slightly hazy
저장 온도
2-8°C
SMILES string
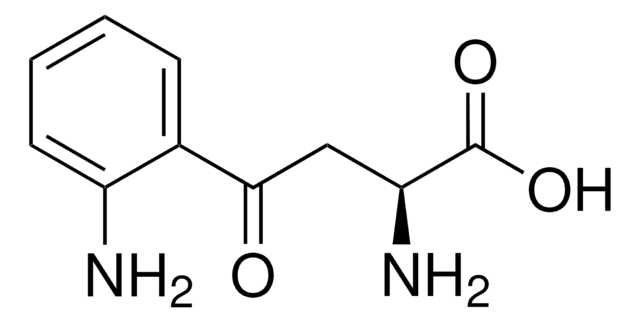
NC(CC(=O)c1cccc(O)c1N)C(O)=O
InChI
1S/C10H12N2O4/c11-6(10(15)16)4-8(14)5-2-1-3-7(13)9(5)12/h1-3,6,13H,4,11-12H2,(H,15,16)
InChI key
VCKPUUFAIGNJHC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
3-Hydroxy-DL-kynurenine is a tryptophan metabolite with a molecular weight corresponding to 224Da. It is a chromophore or hydrophilic yellow compound present in the lens of the eye. 3-Hydroxy-DL-kynurenine is synthesized from kynurenine by the action of enzyme kynurenine 3-monooxygenase (KMO). It is metabolized to 3-hydroxyanthranilic acid and xanthurenic acid in the presence of enzymes kynureninase and kynurenine aminotransferases, respectively.
애플리케이션
3-Hydroxy-DL-kynurenine has been used:
- as a substrate for the recombinant human kynureninase assay
- as a reference standard in tandem mass spectrometry (MS/MS) analysis
- as a control for quantifying serum 3-Hydroxy-DL-kynurenine levels in diabetic retinopathy patients
생화학적/생리학적 작용
3-Hydroxy-DL-kynurenine (3-HKYN) has antioxidant functionality and prevents lipid peroxidation in cerebral cortex. It may modulate synaptic neurotransmission. The levels of 3-HKYN is elevated in Huntington′s disease. However, its role in neurodegenerative diseases is still not understood well. Kynurenine metabolism is linked with glutamatergic neurotransmission and the level of 3-HKYN is linked to the pathophysiology of schizophrenia.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Susanna Campesan et al.
Current biology : CB, 21(11), 961-966 (2011-06-04)
Neuroactive metabolites of the kynurenine pathway (KP) of tryptophan degradation have been implicated in the pathophysiology of neurodegenerative disorders, including Huntington's disease (HD) [1]. A central hallmark of HD is neurodegeneration caused by a polyglutamine expansion in the huntingtin (htt)
A M Myint et al.
Brain, behavior, and immunity, 25(8), 1576-1581 (2011-05-31)
The association between the pro-inflammatory state of schizophrenia and increased tryptophan degradation into kynurenine has been reported. However, the relationship between metabolites from subdivisions of the kynurenine pathway, kynurenic acid and 3-hydroxykynurenine, remains unknown. The present study tested the relationship
Yuri P Tsentalovich et al.
Investigative ophthalmology & visual science, 52(10), 7687-7696 (2011-08-30)
To compare the photochemical properties of UV filter molecules present in the human lens (kynurenine, KN; 3-hydroxykynurenine, 3OHKN; 3-hydroxykynurenine O-β-D-glucoside, 3OHKG; 4-(2-aminophenyl)-4-oxobutanoic acid, AHA; and glutathionyl-kynurenine, GSH-KN) with the use of the following parameters: excited singlet lifetime τ(S), fluorescence quantum
Ruth Condray et al.
The international journal of neuropsychopharmacology, 14(6), 756-767 (2011-01-29)
One branch of the tryptophan catabolic cascade is the kynurenine pathway, which produces neurotoxic [3-hydroxykynurenine (3-OHKY), quinolinic acid] and neuroinhibitory (kynurenic acid) compounds. Kynurenic acid acts as a competitive antagonist at the glycine site of N-methyl-d-asparate receptors at high concentrations
Sarah S Zaher et al.
Investigative ophthalmology & visual science, 52(5), 2640-2648 (2011-01-08)
IDO (indoleamine 2,3-dioxygenase) modulates the immune response by depletion of the essential amino acid tryptophan, and IDO overexpression has been shown to prolong corneal allograft survival. This study was conducted to examine the effect of kynurenines, the products of tryptophan
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.