추천 제품
제품명
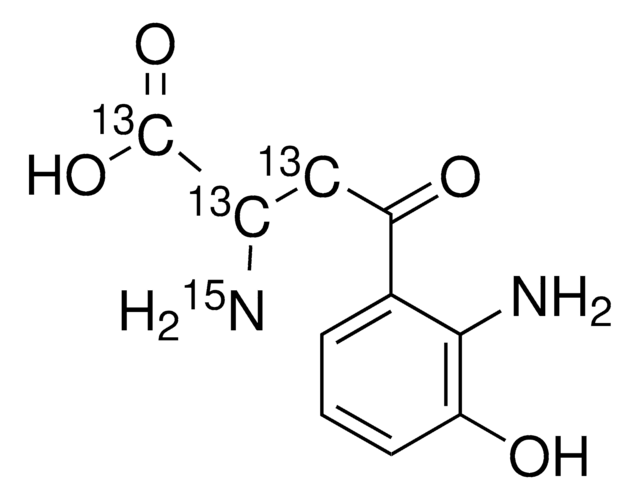
3-Hydroxyanthranilic acid, 97%
분석
97%
양식
solid
반응 적합성
reaction type: solution phase peptide synthesis
mp
240 °C (dec.) (lit.)
응용 분야
peptide synthesis
SMILES string
Nc1c(O)cccc1C(O)=O
InChI
1S/C7H7NO3/c8-6-4(7(10)11)2-1-3-5(6)9/h1-3,9H,8H2,(H,10,11)
InChI key
WJXSWCUQABXPFS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
3-Hydroxyanthranilic acid is an aminobenzoic acid commonly used as an intermediate in various chemical reactions.
애플리케이션
<ul>
<li><strong>Direct enzyme inhibition to mitigate quinolinic acid formation:</strong> Used to synthesize 2-amino-3-carboxymuconic semialdehyde (ACMS) during the kynurenine pathway of Tryptophan catabolism (Sanz et al., 2022).</li>
</ul>
<li><strong>Direct enzyme inhibition to mitigate quinolinic acid formation:</strong> Used to synthesize 2-amino-3-carboxymuconic semialdehyde (ACMS) during the kynurenine pathway of Tryptophan catabolism (Sanz et al., 2022).</li>
</ul>
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Guifang Gan et al.
Cell death discovery, 7(1), 173-173 (2021-07-08)
Sorafenib is the FDA-approved first-line target drug for HCC patients. However, sorafenib only confers 3-5 months of survival benefit with <30% of HCC patients. Thus, it is necessary to develop a sensitizer for hepatocellular carcinoma (HCC) to sorafenib. Here, we
Andrea Moglia et al.
Metabolic engineering, 12(3), 223-232 (2009-11-28)
Phenolic esters like chlorogenic acid play an important role in therapeutic properties of many plant extracts. We aimed to produce phenolic esters in baker's yeast, by expressing tobacco 4CL and globe artichoke HCT. Indeed yeast produced phenolic esters. However, the
Raf Brouns et al.
Neurochemical research, 35(9), 1315-1322 (2010-05-22)
Post-stroke inflammation may induce upregulation of the kynurenine (KYN) pathway for tryptophan (TRP) oxidation, resulting in neuroprotective (kynurenic acid, KA) and neurotoxic metabolites (3-hydroxyanthranillic acid, 3-HAA). We investigated whether activity of the kynurenine pathway in acute ischemic stroke is related
Vilma Gabbay et al.
Journal of child psychology and psychiatry, and allied disciplines, 51(8), 935-943 (2010-04-22)
Although adolescent major depressive disorder (MDD) is acknowledged to be a heterogeneous disorder, no studies have reported on biological correlates of its clinical subgroups. This study addresses this issue by examining whether adolescent MDD with and without melancholic features (M-MDD
Vilma Gabbay et al.
Progress in neuro-psychopharmacology & biological psychiatry, 34(1), 37-44 (2009-09-26)
Cytokine induction of the enzyme indoleamine 2,3-dioxygenase (IDO) has been implicated in the development of major depressive disorder (MDD). IDO metabolizes tryptophan (TRP) into kynurenine (KYN), thereby decreasing TRP availability to the brain. KYN is further metabolized into several neurotoxins.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.