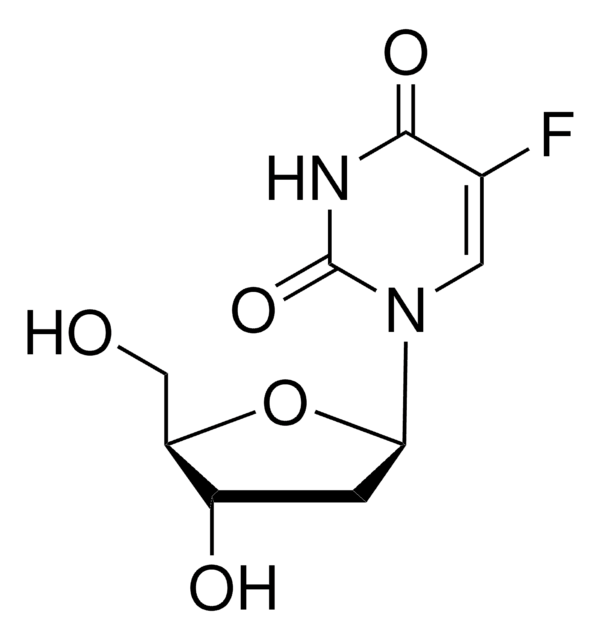
F5130
5-Fluorouridine
proapoptotic anitproliferative plant growth regulator
동의어(들):
5-Fluorouracil 1β-D-ribofuranoside, FUrd
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
실험식(Hill 표기법):
C9H11FN2O6
CAS Number:
Molecular Weight:
262.19
Beilstein:
33662
EC Number:
MDL number:
UNSPSC 코드:
41106305
PubChem Substance ID:
NACRES:
NA.51
추천 제품
생물학적 소스
synthetic (organic)
분석
≥99% (HPLC)
형태
powder
solubility
water: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
SMILES string
OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N2C=C(F)C(=O)NC2=O
InChI
1S/C9H11FN2O6/c10-3-1-12(9(17)11-7(3)16)8-6(15)5(14)4(2-13)18-8/h1,4-6,8,13-15H,2H2,(H,11,16,17)/t4-,5-,6-,8-/m1/s1
InChI key
FHIDNBAQOFJWCA-UAKXSSHOSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
5-Fluorouridine (FUrd) is a fluoropyrimidine nucleoside analog and a cell-permeable modified RNA precursor.
애플리케이션
5-Fluorouridine has been used for labeling active transcription sites in the porcine fetal fibroblasts and human cell lines for immunocytochemistry analysis. It has also been used to monitor apoptosis during drug sensitivity assay in esophageal squamous cell carcinoma (ESCC) cells.
생화학적/생리학적 작용
5-Fluorouridine (FUrd) is cytotoxic towards cancer cells. FUrd is often used in chemical and biochemical comparison studies with fluorouracil and thymine analogs.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Inhibition of RNA synthesis by 5-fluorouridine accounts for its cyto toxicity on colorectal cancer cells in vitro
Subbarayan PR, et al.
Cancer research, 65(9) (2005)
P V Sahasrabudhe et al.
Nucleic acids research, 23(19), 3916-3921 (1995-10-11)
The effects of 5-fluorouridine (FUrd) and 5-fluorodeoxyuridine (FdUrd) substitution on the stabilities of duplex RNA and DNA have been studied to determine how FUrd substitution in nucleic acids may alter the efficiency of biochemical processes that require complementary base pairing
Angelica M Bello et al.
Journal of medicinal chemistry, 52(6), 1648-1658 (2009-03-06)
A series of 6-substituted and 5-fluoro-6-substituted uridine derivatives were synthesized and evaluated for their potential as anticancer agents. The designed molecules were synthesized from either fully protected uridine or the corresponding 5-fluorouridine derivatives. The mononucleotide derivatives were used for enzyme
Edward J Miracco et al.
Journal of the American Chemical Society, 133(31), 11826-11829 (2011-07-13)
The pseudouridine synthase TruB handles 5-fluorouridine in RNA as a substrate, converting it into two isomeric hydrated products. Unexpectedly, the two products differ not in the hydrated pyrimidine ring but in the pentose ring, which is epimerized to arabinose in
Iñigo Casafont et al.
Neurotoxicity research, 17(2), 167-178 (2009-07-18)
The ubiquitin-dependent proteasome system (UPS) is the major pathway responsible for selective nuclear and cytoplasmic protein degradation. Bortezomib, a boronic acid dipeptide, is a reversible 20S proteasome inhibitor used as novel anticancer drug, particularly in the treatment of multiple myeloma
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.