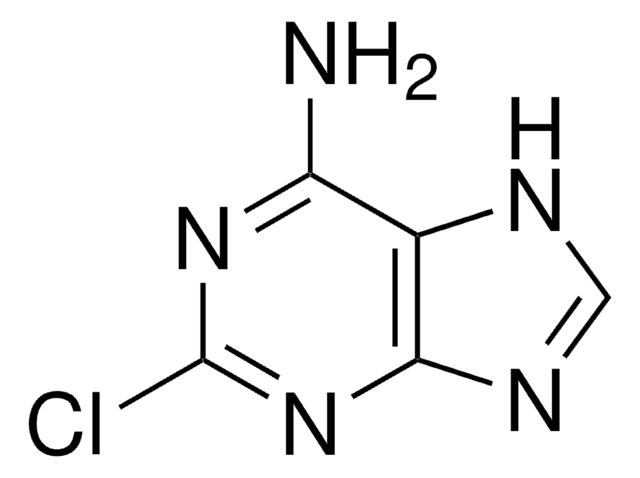
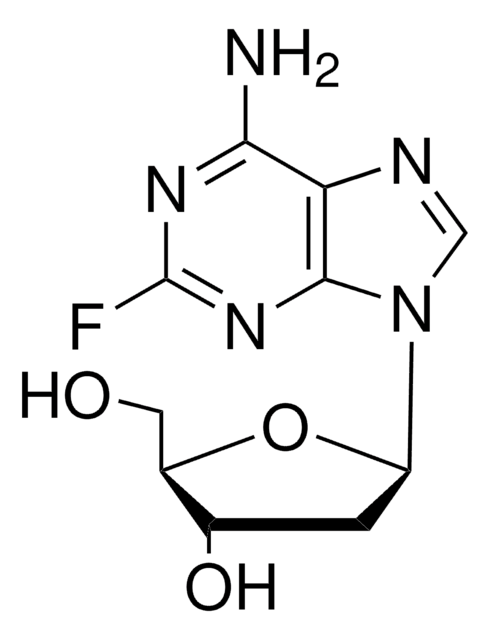
추천 제품
Quality Level
분석
97%
형태
solid
mp
240 °C (D) (lit.)
SMILES string
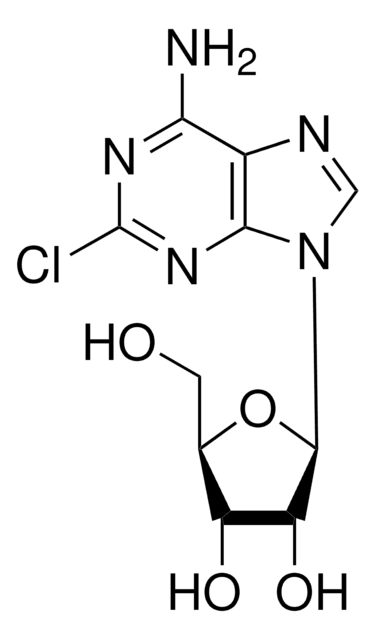
Nc1nc(F)nc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1
InChI key
HBUBKKRHXORPQB-UUOKFMHZSA-N
유전자 정보
human ... ADORA3(140)
rat ... Adora1(29290) , Adora2a(25369) , Adora3(25370)
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
U K Decking et al.
The American journal of physiology, 266(4 Pt 2), H1596-H1603 (1994-04-01)
Transport and phosphorylation of 2-fluoroadenosine (F-AR) were studied in human erythrocytes and porcine aortic endothelial cells by 19F-nuclear magnetic resonance (NMR) spectroscopy. F-AR (590 microM) added to a human erythrocyte suspension (15% hematocrit) was rapidly incorporated into adenine nucleotides at
M L Deras et al.
Biochemistry, 38(1), 303-310 (1999-01-16)
In contrast to several other glutamine amidotransferases including asparagine synthetase, cytidine 5'-triphosphate (CTP) synthetase, carbamoyl phosphate synthetase, and phosphoribosyl pyrophosphate (PRPP) amidotransferase, guanosine monophosphate synthetase (GMPS) will not utilize hydroxylamine as an alternative nitrogen source. Instead, the enzyme is inhibited
Larissa Romanello et al.
Acta crystallographica. Section D, Biological crystallography, 69(Pt 1), 126-136 (2013-01-01)
In adult schistosomes, the enzyme adenosine kinase (AK) is responsible for the incorporation of some adenosine analogues, such as 2-fluoroadenosine and tubercidin, into the nucleotide pool, but not others. In the present study, the structures of four complexes of Schistosoma
V B Berzin et al.
Bioorganicheskaia khimiia, 35(2), 210-214 (2009-06-20)
The preparative method for the synthesis of 2-fluoroadenosine starting from commercially available guanosine was developed. It included the intermediate formation of 2-amino-6-azido-9-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)purine, which was isolated exclusively in the tetrazolo[5,1-i]-form {5-amino-7-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)-7H-tetrazolo[5,1-i]purine}. The latter compound was converted by the Schiemann reaction to
Mian M Alauddin et al.
Nuclear medicine and biology, 34(3), 267-272 (2007-03-27)
Many fluorinated analogues of adenosine nucleoside have been synthesized and studied as potential antitumor and antiviral agents. Earlier, we reported radiosynthesis of 2'-deoxy-2'-[(18)F]fluoro-1-beta-D-arabinofuranosyl-adenine ([(18)F]-FAA) and 3'-deoxy-3'-[(18)F]fluoro-1-beta-d-xylofuranosyl-adenine ([(18)F]FXA). Now, we report their in vivo studies including blood clearance, biodistribution and micro-PET
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.