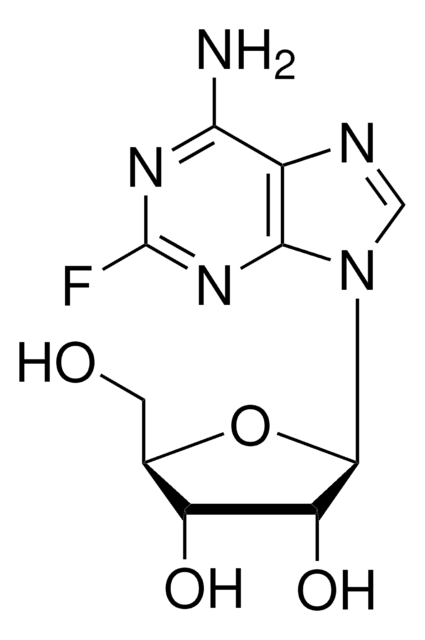
F2773
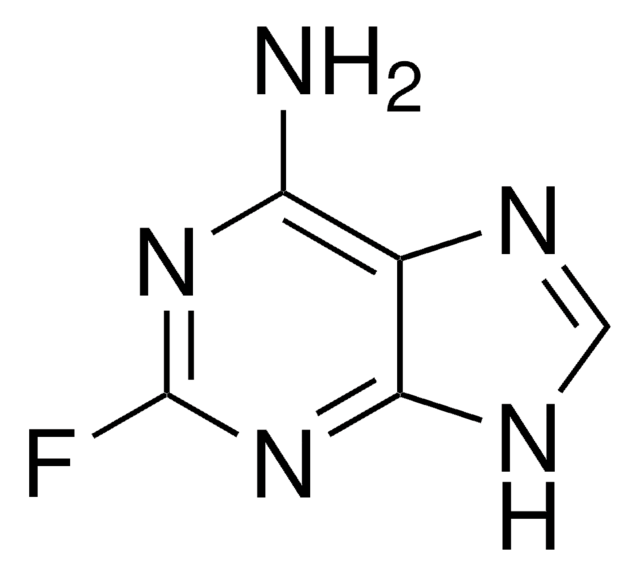
2-Fluoroadenine-9-β-D-arabinofuranoside
DNA synthesis and methylation inhibitor
동의어(들):
9-β-D-Arabinofuranosyl-2-fluoroadenine, F-ara-A, Fludarabine des-phosphate
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C10H12FN5O4
CAS Number:
Molecular Weight:
285.23
Beilstein:
1225932
EC Number:
MDL number:
UNSPSC 코드:
12352202
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
저장 온도
2-8°C
SMILES string
Nc1nc(F)nc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O
InChI
1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6+,9-/m1/s1
InChI key
HBUBKKRHXORPQB-FJFJXFQQSA-N
유전자 정보
human ... ADORA3(140)
rat ... Adora1(29290) , Adora2a(25369) , Adora3(25370)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
2-Fluoroadenine-9-β-D-arabinofuranoside (F-ara-A) has been used:
- to assess its interaction with kinase inhibitor UCN-01 in human leukemia cells (U937 and HL-60) and primary patient samples
- to assess its influence on endothelial cells by apoptosis assay and human microvascular endothelial cells (HMECs) by cytotoxicity assay
- in comparative cytotoxicity studies with Alemtuzumab CDC in 17p13- chronic lymphocytic leukemia (CLL) patients
생화학적/생리학적 작용
Fludarabine (the 5′-phosphate) is a prodrug that is converted to F-ara-A, which enters cells and accumulates primarily as the 5′-triphosphate. F-ara-A interferes with DNA synthesis and repair and induces apoptosis of cancer cells.
Fludarabine (the 5′-phosphate) is a prodrug that is converted to F-ara-A, which enters cells and accumulates primarily as the 5′-triphosphate. F-ara-A interferes with DNA synthesis and repair and induces apoptosis of cancer cells. F-ara-A also strongly inhibits DNA methylation, particularly methylation of cytosine in CpG dinucleotide sequences.
경고
The name fludarabine refers to 9-β-D-arabinofuranosyl-2-fluoroadenine 5′-phosphate, but is sometimes erroneously used for this compound, which lacks the phosphate.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Muta. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide
Eissner G, et al.
Blood, 100(1), 334-340 (2002)
Alessandro Natoni et al.
Methods in molecular biology (Clifton, N.J.), 986, 217-226 (2013-02-26)
Chronic Lymphocytic Leukaemia (CLL) is an incurable disease that warrants new therapeutic treatments. CLL cells accumulate in the peripheral blood, in the bone marrow and in secondary lymphoid organs. Unlike circulating CLL cells, CLL cells resident in these last two
Valentina Griggio et al.
Haematologica, 105(4), 1042-1054 (2019-07-11)
In chronic lymphocytic leukemia (CLL), the hypoxia-inducible factor 1 (HIF-1) regulates the response of tumor cells to hypoxia and their protective interactions with the leukemic microenvironment. In this study, we demonstrate that CLL cells from TP53-disrupted (TP53dis) patients have constitutively
Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early-intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab
Zent CS, et al.
Leukemia Research, 32(12), 1849-1856 (2008)
M J Keating et al.
Blood, 74(1), 19-25 (1989-07-01)
Fludarabine was used to treat 68 patients with previously treated chronic lymphocytic leukemia (CLL). Nine (13%) patients achieved a complete remission and 30 (44%) a partial remission. The response rates for Rai stages 0 to 2, 3, and 4 were
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.