추천 제품
생물학적 소스
microbial
Quality Level
분석
≥75%
양식
powder
색상
white to off-white
mp
156-158 °C (lit.)
저장 온도
2-8°C
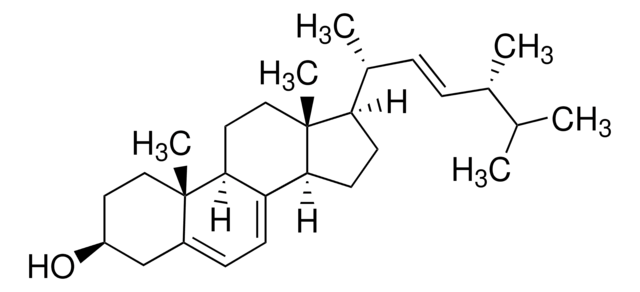
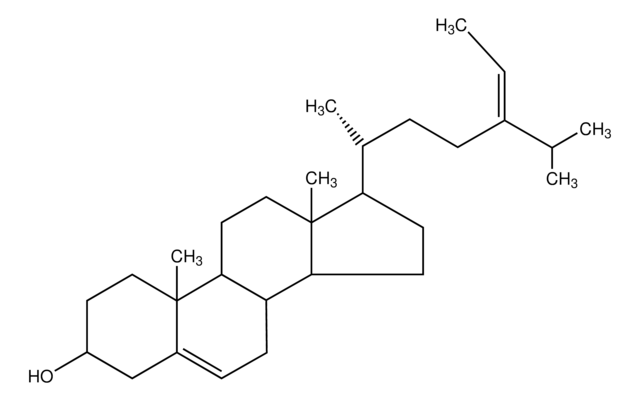
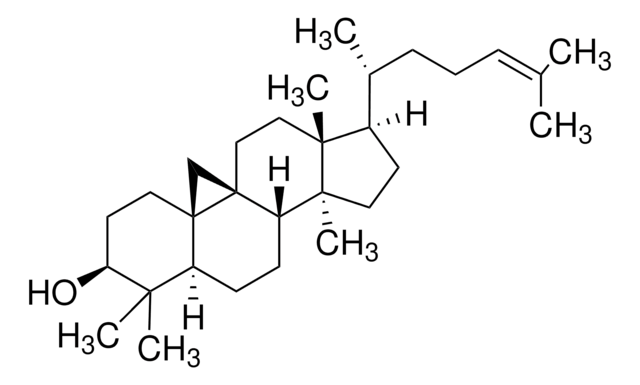
SMILES string
[H][C@@]1(CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)\C=C\[C@H](C)C(C)C
InChI
1S/C28H44O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h7-10,18-20,22,24-26,29H,11-17H2,1-6H3/b8-7+/t19-,20+,22-,24+,25-,26-,27-,28+/m0/s1
InChI key
DNVPQKQSNYMLRS-APGDWVJJSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
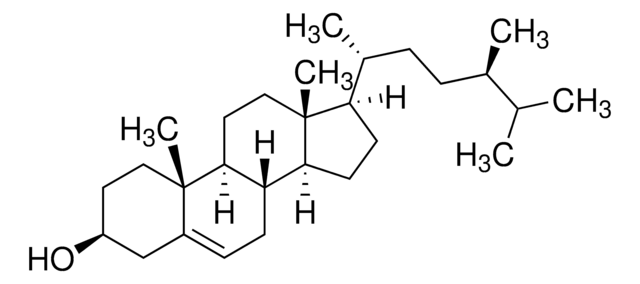
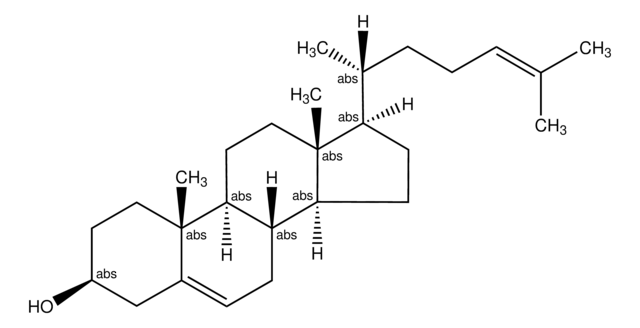
일반 설명
애플리케이션
- as a component of yeast cell monolayer models, to study the effects of steroidal and triterpenoid saponins on the monolayers
- as a standard sample, to isolate and identify a fungal metabolite fraction containing ergosterol
- as a component of the culture medium to isolate the CYP51RNAi phenotype of Trypanosoma brucei gambiense, to verify ergosterol biosynthesis
생화학적/생리학적 작용
이미 열람한 고객
문서
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.