추천 제품
생물학적 소스
rabbit
Quality Level
결합
unconjugated
항체 형태
affinity isolated antibody
항체 생산 유형
primary antibodies
클론
polyclonal
양식
buffered aqueous solution
분자량
antigen ~22 kDa
종 반응성
rat, hamster, monkey, mouse, bovine, human, canine
농도
~1 mg/mL
기술
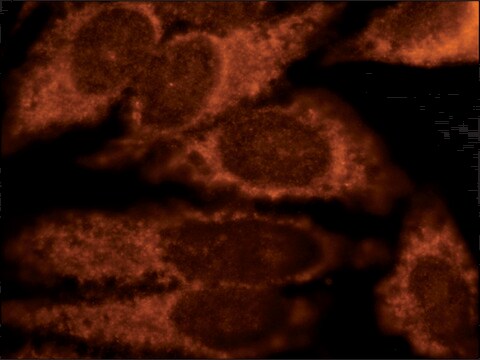
indirect immunofluorescence: 2.5-5 μg/mL using rat NRK cells
western blot (chemiluminescent): 0.2-0.4 μg/mL using whole extract of human HeLa and mouse 3T3 cells.
UniProt 수납 번호
배송 상태
dry ice
저장 온도
−20°C
타겟 번역 후 변형
unmodified
유전자 정보
human ... DERL1(79139)
mouse ... Derl1(67819)
일반 설명
Derlin-1 is a 22kDa hydrophobic protein that spans the lipid bilayer of the ER four times with its amino- and carboxy-terminus in the cytosol. It is expressed with high levels in liver, spleen, pancreas, lung, thymus, and ovary.
Derlin-1 shares human homology with yeast Der1p.
면역원
a synthetic peptide corresponding to the C-terminal region of human Derlin-1 with N-terminal added cysteine, conjugated to KLH. The corresponding sequence is identical in mouse.
애플리케이션
Anti-Derlin-1 antibody produced in rabbit has been used in:
- immunostaining
- co-immunoprecipitation
- immunofluorescence
생화학적/생리학적 작용
Derlin-1 can interact with peptide:N-glycanase (PNGase), a deglycosylating enzyme, bringing it close to misfolding dislocating glycoproteins.
Derlin-1 is required for the dislocation of misfolded proteins from the ER lumen to the cytosol, where they are destroyed by the ubiquitin-proteasome system. It interacts with PNGase, a deglycosylating enzyme, bringing it close to misfolding dislocating glycoproteins. It forms a membrane protein complex with VIMP ( (VCP-interacting membrane protein) and this complex serves as a receptor for p97. p97 interacts with several ubiquitin ligases, thus recruiting them to Derlin-1.
물리적 형태
Solution in 0.01 M phosphate buffered saline, pH 7.4, containing 15 mM sodium azide.
면책조항
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Zlatka Kostova et al.
The EMBO journal, 22(10), 2309-2317 (2003-05-14)
The surveillance of the structural fidelity of the proteome is of utmost importance to all cells. The endoplasmic reticulum (ER) is the organelle responsible for proper folding and delivery of proteins to the secretory pathway. It contains a sophisticated protein
Molecular characterization and expression of DERL1 in bovine ovarian follicles and corpora lutea
Ndiaye K, et al.
Reproductive Biology and Endocrinology, 8(1), 94-94 (2010)
Yihong Ye et al.
Proceedings of the National Academy of Sciences of the United States of America, 102(40), 14132-14138 (2005-09-28)
Misfolded proteins are eliminated from the endoplasmic reticulum (ER) by retrotranslocation into the cytosol, a pathway hijacked by certain viruses to destroy MHC class I heavy chains. The translocation of polypeptides across the ER membrane requires their polyubiquitination and subsequent
Yihong Ye et al.
Nature, 429(6994), 841-847 (2004-06-25)
Elimination of misfolded proteins from the endoplasmic reticulum (ER) by retro-translocation is an important physiological adaptation to ER stress. This process requires recognition of a substrate in the ER lumen and its subsequent movement through the membrane by the cytosolic
Brendan N Lilley et al.
Nature, 429(6994), 834-840 (2004-06-25)
After insertion into the endoplasmic reticulum (ER), proteins that fail to fold there are destroyed. Through a process termed dislocation such misfolded proteins arrive in the cytosol, where ubiquitination, deglycosylation and finally proteasomal proteolysis dispense with the unwanted polypeptides. The
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.