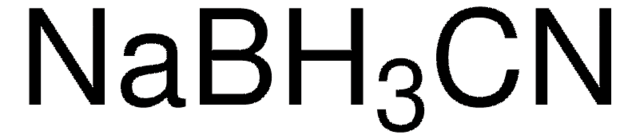추천 제품
양식
liquid
Quality Level
반응 적합성
reagent type: reductant
기술
affinity chromatography: suitable
응용 분야
life science and biopharma
저장 온도
2-8°C
애플리케이션
A ready-to-use reagent used to couple amine ligands to aldehyde functional groups. The coupling buffer reaction is a reductive amination of the intermediate Schiff′s base to a stable C−N bond.
Cyanoborohydride Coupling Buffer has been used:
- in coupling reactions between amines and glutaraldehyde
- to reduce hydrazone bond to a stable hydrazide bond
- as a component in oligonucleotide reaction mixture for coverslips functionalization
Cyanoborohydride Coupling Buffer is used in affinity chromatography, protein chromatography, activated/functionalized matrices and synthetic reagents. Cyanoborohydride has been used to inform a safe and effective gene-transfer system targeting hepatocytes as well as to develop a method for targeted delivery of anticancer therapeutics to cancer cells in hypoxic areas.
생화학적/생리학적 작용
Cyanoborohydride Coupling Buffer is a reagent suitable for reductive amination processes, that contributes to transformation of simple alcohols into more complex amines. It is used in the conversion of Schiff base, by reducing it, to form a secondary amine without affecting aldehyde groups on the support.
성분
0.02 M sodium phosphate, pH 7.5, containing 0.2 M sodium chloride and 3.0 g/L sodium cyanoborohydride
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Use of polymer supported reagents for clean multi-step organic synthesis: preparation of amines and amine derivatives from alcohols for use in compound library generation
Ley S, et al.
Journal of the Chemical Society. Perkin Transactions 1, 15(27), 2239-2242 (1998)
Christopher A Holden et al.
International journal of nanomedicine, 5, 25-36 (2010-02-18)
Tumors frequently contain hypoxic regions that result from a shortage of oxygen due to poorly organized tumor vasculature. Cancer cells in these areas are resistant to radiation- and chemotherapy, limiting the treatment efficacy. Macrophages have inherent hypoxia-targeting ability and hold
Direct electrical detection of antigen?antibody binding on diamond and silicon substrates using electrical impedance spectroscopy.
Yang W, et al.
Analyst, 132(4), 296-306 (2007)
Binding between the integrin ?X?2 (CD11c/CD18) and heparin.
Vorup-Jensen T, et al.
Test, 282(42), 30869-30877 (2007)
Polymer-supported triacetoxyborohydride: a novel reagent of choice for reductive amination
Bhattacharyya S, et al.
Tetrahedron Letters, 44(27), 4957-4960 (2003)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.