B4563
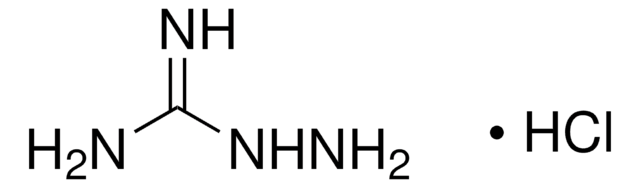
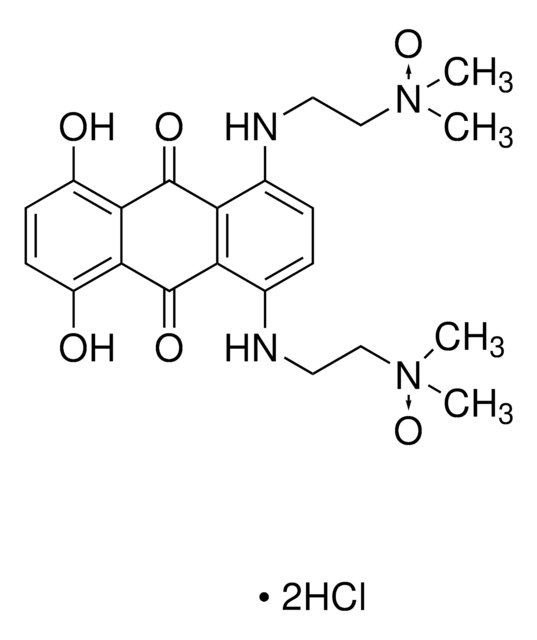
Bisantrene dihydrochloride
≥98% (HPLC)
동의어(들):
Bis((4,5-dihydro-1H-imidazol-2-yl)hydrazone)-9,10-anthracenedicarboxaldehyde dihydrochloride, NSC 337766
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C22H22N8 ·2HCl
CAS Number:
Molecular Weight:
471.39
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
solid
저장 조건
desiccated
solubility
deionized water: 8 mg/mL
저장 온도
room temp
SMILES string
Cl.Cl.C1CN=C(N1)N\N=C\c2c3ccccc3c(\C=N\NC4=NCCN4)c5ccccc25
InChI
1S/C22H22N8.2ClH/c1-2-6-16-15(5-1)19(13-27-29-21-23-9-10-24-21)17-7-3-4-8-18(17)20(16)14-28-30-22-25-11-12-26-22;;/h1-8,13-14H,9-12H2,(H2,23,24,29)(H2,25,26,30);2*1H/b27-13+,28-14+;;
InChI key
KINULKKPVJYRON-PVNXHVEDSA-N
애플리케이션
Bisantrene (NSC 337766), and its derivatives are topoisomerase II poisons and DNA intercalators. It may be used as model compounds to study P-glycoprotein-mediated multiple drug resistance (MDR1). Bisantrene may be used as a Rac1 inhibitor.
생화학적/생리학적 작용
Bisantrene dihydrochloride is an antineoplastic agent, MDR1 substrate, DNA intercalator, and topoisomerase II poison. Cancer cells that develop resistance to bisantrene tend to overexpress P-glycoprotein. Bisantrene can be used to select for P-glycoprotein-mediated multiple drug resistance. The data suggest that bisantrene is an excellent substrate for P-glycoprotein.
Bisantrene is an anthracene bishydrazone derivative, which has antitumor activity.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
G Capranico et al.
Journal of molecular biology, 235(4), 1218-1230 (1994-01-28)
To gain further knowledge of the molecular features of topoisomerase II inhibitors required for drug-receptor complex formation, we investigated the conformational drug determinants of the sequence specificities of drug-stimulated DNA cleavage by computer-aided molecular modeling techniques. DNA sequence specificities of
T P Wunz et al.
Journal of medicinal chemistry, 33(6), 1549-1553 (1990-06-01)
The relative DNA binding strengths of bisantrene and nine new analogues were measured by spectrophotometric titration and melt transition temperature (Tm) techniques. Data from the spectrophotometric titrations could not be fit by simple Scatchard plots. However, they were fit by
K C Murdock et al.
Journal of medicinal chemistry, 36(15), 2098-2101 (1993-07-23)
The selective phosphorylation of bisantrene (1) affords bis(phosphonoguanidinic acid) 6, a prodrug with enhanced aqueous solubility (as sodium salt 7) at physiological pH. Unlike 1, in a rat tail vein model, no precipitation was observed when bis(phosphonoguanidinic acid) 6 was
A Spadea et al.
Leukemia & lymphoma, 9(3), 217-220 (1993-02-01)
Because of the lack of standard treatment in refractory and relapsed acute myelogenous leukemia (AML) several new drugs have been employed alone to evaluate their efficacy in this peculiar category of patient. Bisantrene, a new anthracene bishydrazone derivative, has shown
G Visani et al.
Haematologica, 75(6), 527-531 (1990-11-01)
In vitro clonogenic assays may be useful for determining the sensitivity of leukemic cells to chemotherapeutic agents. We evaluated the antileukemic effect of Bisantrene (an anthracene derivative now undergoing phase II clinical trials in relapsed/resistant acute non lymphoid leukemias-ANLL) using
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.