B1532
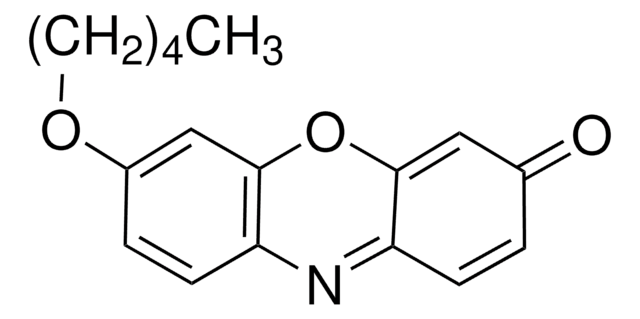
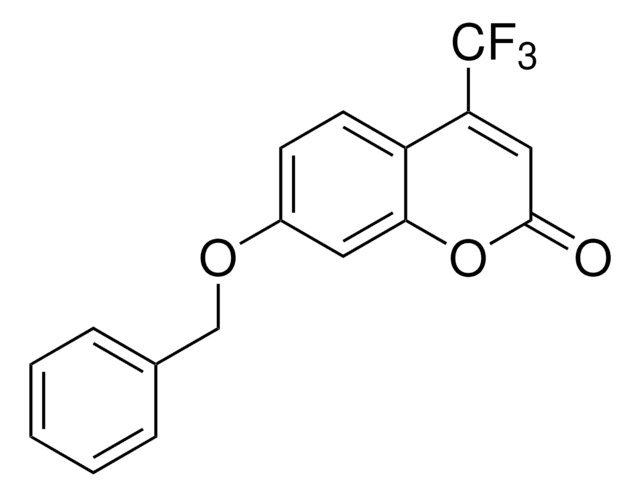
Resorufin benzyl ether
CYP450 substrate, ≥98% (TLC), powder
동의어(들):
7-Benzyloxy-3H-phenoxazin-3-one, 7-Benzyloxyresorufin, O7-Benzylresorufin
About This Item
추천 제품
제품명
Resorufin benzyl ether, CYP450 substrate
Quality Level
분석
≥98% (TLC)
양식
powder
solubility
chloroform: 9.80-10.20 mg/mL, clear, orange
저장 온도
2-8°C
SMILES string
O=C1C=CC2=Nc3ccc(OCc4ccccc4)cc3OC2=C1
InChI
1S/C19H13NO3/c21-14-6-8-16-18(10-14)23-19-11-15(7-9-17(19)20-16)22-12-13-4-2-1-3-5-13/h1-11H,12H2
InChI key
XNZRYTITWLGTJS-UHFFFAOYSA-N
기질
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
문서
Phase I biotransformation reactions introduce or expose functional groups on the drug with the goal of increasing the polarity of the compound. Although Phase I drug metabolism occurs in most tissues, the primary and first pass site of metabolism occurs during hepatic circulation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.