추천 제품
생물학적 소스
synthetic (organic)
Quality Level
무균
non-sterile
양식
powder
solubility
DMSO: soluble 14 mg/mL at ≤60 °C
H2O: insoluble
배송 상태
ambient
저장 온도
room temp
SMILES string
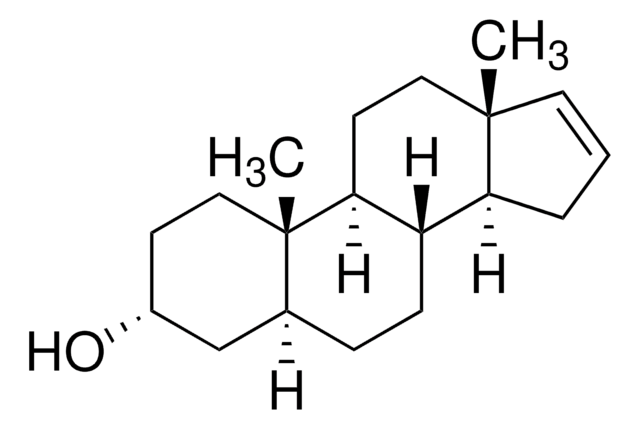
[H][C@@]12CC[C@@]3([H])[C@]4([H])CCC[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@H](O)C2
InChI
1S/C19H32O/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18/h13-17,20H,3-12H2,1-2H3/t13-,14-,15-,16-,17-,18-,19-/m0/s1
InChI key
DJTOLSNIKJIDFF-LOVVWNRFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
5α-Androstan-3β-ol has been used as a cortisone analog to test its effect on the voltage-dependent potassium channel (Kv) current.
생화학적/생리학적 작용
mCAR (constitutive androstane receptor) inverse agonist; testosterone metabolite.
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 4
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
A Kassam et al.
The Journal of biological chemistry, 275(6), 4345-4350 (2000-02-08)
The genes encoding the first two enzymes of the peroxisomal beta-oxidation pathway, acyl-CoA oxidase (AOx) and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase (HD), contain upstream cis-acting regulatory regions termed peroxisome proliferator response elements (PPRE). Transcription of these genes is mediated through the binding
I Tzameli et al.
Molecular and cellular biology, 20(9), 2951-2958 (2000-04-11)
A wide range of xenobiotic compounds are metabolized by cytochrome P450 (CYP) enzymes, and the genes that encode these enzymes are often induced in the presence of such compounds. Here, we show that the nuclear receptor CAR can recognize response
Isabelle Dussault et al.
Molecular and cellular biology, 22(15), 5270-5280 (2002-07-09)
Unlike classical nuclear receptors that require ligand for transcriptional activity, the constitutive androstane receptor (CAR) is active in the absence of ligand. To determine the molecular contacts that underlie this constitutive activity, we created a three-dimensional model of CAR and
Jorge Luiz Vieira Dos Anjos et al.
International journal of pharmaceutics, 345(1-2), 81-87 (2007-06-30)
The interaction of a potent percutaneous penetration enhancer, 1,8-cineole, with the stratum corneum (SC) and DPPC membranes was investigated by electron paramagnetic resonance spectroscopy (EPR) of spin-labeled analogs of stearic acid (5-DSA) and androstanol (ASL). The EPR spectra of lipid
Andrea Toell et al.
Journal of cellular biochemistry, 85(1), 72-82 (2002-03-14)
Constitutive androstane receptor (CAR) and pregnane X receptor (PXR) are members of the nuclear receptor superfamily that regulate target gene transcription in a ligand-dependent manner. CAR and PXR have a rather broad, overlapping set of ligands that range from natural
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.