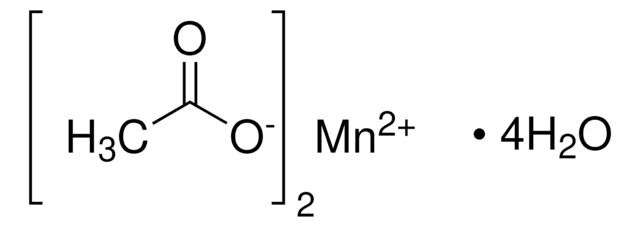
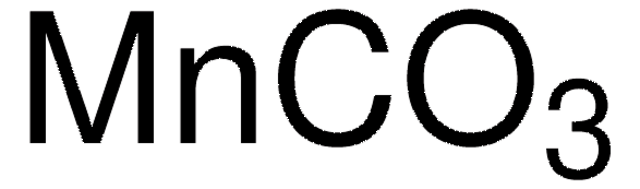추천 제품
vapor pressure
<1 hPa ( 25 °C)
Quality Level
grade
purum p.a.
분석
≥99.0% (KT)
양식
crystals
pH
7 (20 °C, 50 g/L)
mp
>300 °C (lit.)
density
1.589 g/mL at 25 °C (lit.)
음이온 미량물
chloride (Cl-): ≤50 mg/kg
sulfate (SO42-): ≤200 mg/kg
양이온 미량물
Ca: ≤50 mg/kg
Cd: ≤50 mg/kg
Co: ≤50 mg/kg
Cu: ≤50 mg/kg
Fe: ≤50 mg/kg
K: ≤100 mg/kg
Na: ≤100 mg/kg
Ni: ≤50 mg/kg
Pb: ≤50 mg/kg
Zn: ≤50 mg/kg
SMILES string
[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(=O)O[Mn]OC(C)=O
InChI
1S/2C2H4O2.Mn.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
InChI key
CESXSDZNZGSWSP-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Manganese(II) acetate tetrahydrate is a hydrated salt of manganese. Kinetic studies suggest that its dehydration takes place in two-step reaction involving the loss of two water molecules. Its crystal structure has been investigated by X-ray studies. Its crystals exhibit monoclinic crystal system and space group P21/c.
애플리케이션
Manganese(II) acetate tetrahydrate may be used in the sol gel synthesis of doped TiO2 containing different amounts of Mn2+.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 3 - STOT RE 2 Inhalation
표적 기관
Brain
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Influence of manganese ions on the anatase-rutile phase transition of TiO2 prepared by the sol-gel process.
Arroyo R, et al.
Materials Letters, 54(5), 397-402 (2002)
Kinetic analysis of thermal decomposition reactions: Part VI. Thermal decomposition of manganese (II) acetate tetrahydrate.
Diefallah EHM.
Thermochimica Acta, 202, 1-16 (1992)
Crystal structure of manganese acetate tetrahydrate.
Bertaut EF, et al.
Acta Crystallographica Section B, Structural Crystallography and Crystal Chemistry, 30(9), 2234-2236 (1974)
Kyu-Nam Jung et al.
Scientific reports, 5, 7665-7665 (2015-01-08)
Rechargeable metal-air batteries are considered a promising energy storage solution owing to their high theoretical energy density. The major obstacles to realising this technology include the slow kinetics of oxygen reduction and evolution on the cathode (air electrode) upon battery
Qiangqiang Sun et al.
Journal of hazardous materials, 286, 276-284 (2015-01-16)
Comparative experiments were conducted to investigate the catalytic ability of MnO(x)/SBA-15 for the ozonation of clofibric acid (CA) and its reaction mechanism. Compared with ozonation alone, the degradation of CA was barely enhanced, while the removal of TOC was significantly
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.