모든 사진(2)
About This Item
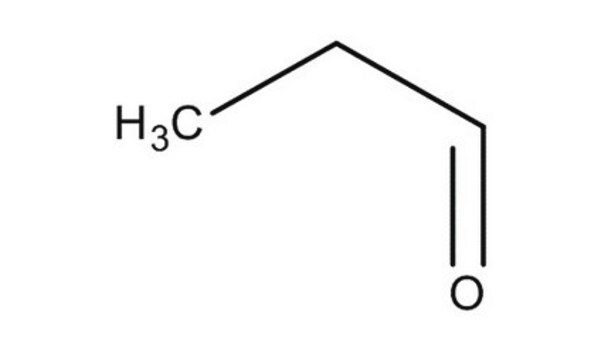
Linear Formula:
CH3CH2CHO
CAS Number:
Molecular Weight:
58.08
Beilstein:
506010
EC Number:
MDL number:
UNSPSC 코드:
12352114
PubChem Substance ID:
NACRES:
NA.21
Grade:
reagent grade
분석:
97%
bp:
46-50 °C (lit.)
vapor pressure:
18.77 psi ( 55 °C)
4.89 psi ( 20 °C)
4.89 psi ( 20 °C)
추천 제품
Grade
reagent grade
Quality Level
vapor density
2 (vs air)
vapor pressure
18.77 psi ( 55 °C)
4.89 psi ( 20 °C)
분석
97%
양식
liquid
autoignition temp.
404 °F
expl. lim.
17 %, 26 °F
2.6 %, 31 °F
불순물
≤2.5% (water)
refractive index
n20/D 1.362 (lit.)
bp
46-50 °C (lit.)
mp
−81 °C (lit.)
solubility
organic solvents: soluble
density
0.805 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
[H]C(=O)CC
InChI
1S/C3H6O/c1-2-3-4/h3H,2H2,1H3
InChI key
NBBJYMSMWIIQGU-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Propionaldehyde, also known as propanal, is commonly utilized as an intermediate in organic reactions. It can be synthesized by the oxidation of propane in gas-phase. Photodissociation behavior of propionaldehyde has been investigated by the Paneth mirror method. The degradation mechanism of propionaldehyde on Rh(111) surface has been analyzed using temperature programmed desorption (TPD) and high resolution electron energy loss spectroscopy (HREELS). 2-Çhloropropionaldehyde has been synthesized from propionaldehyde, via electrochemical chlorination. The presence of two stable rotamers in propionaldehyde has been reported based on microwave spectral data.
애플리케이션
- Unraveling the electrocatalytic reduction mechanism of enols on copper in aqueous media.: Research on the electrocatalytic reduction mechanisms of enols, including implications for propionaldehyde, provides valuable information for chemical synthesis and industrial applications in catalysis (Cui et al., 2022).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
-22.0 °F - closed cup
Flash Point (°C)
-30 °C - closed cup
이미 열람한 고객
Reactions of 1-propanol and propionaldehyde on rhodium (111).
Brown NF and Barteau MA.
Langmuir, 8(3), 862-869 (1992)
Mechanism of the Photolysis of Propionaldehyde.
Garrison WM and Burton M.
J. Chem. Phys., 10(12), 730-739 (1942)
Eagleson M.
Concise Encyclopedia Chemistry, 898-898 (1994)
Microwave spectrum of propionaldehyde.
Butcher SS and Wilson Jr EB.
J. Chem. Phys. , 40(6), 1671-1678 (1964)
Chlorination of propionaldehyde by electrolysis of aqueous chloride solutions.
Maksimov KHA, et al.
Russian Journal of Applied Chemistry, 68(4), 524-526 (1995)
프로토콜
-Tolualdehyde; Valeraldehyde; Isovaleraldehyde
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.