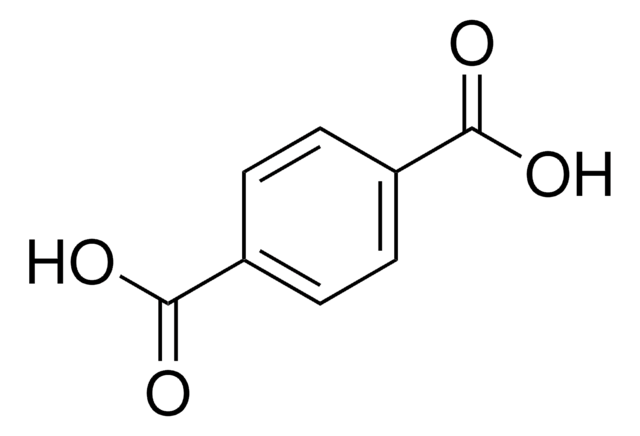
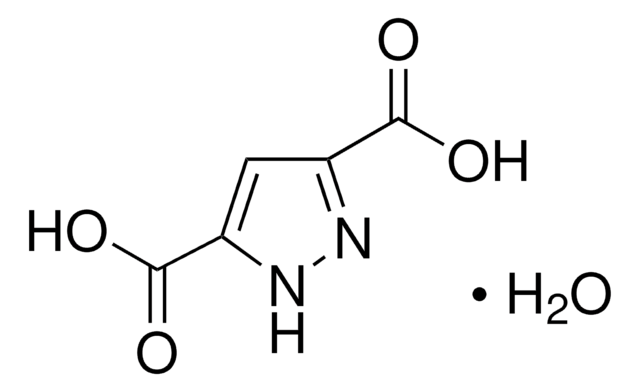
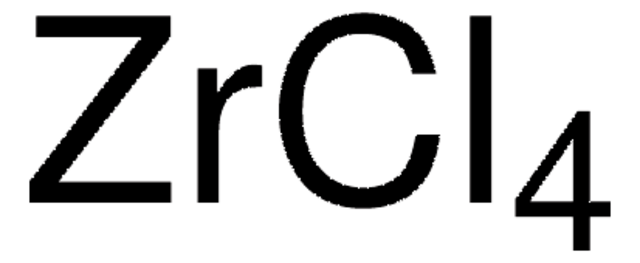추천 제품
Grade
reagent
vapor pressure
1 mmHg ( 100 °C)
제품 라인
ReagentPlus®
분석
99%
양식
crystalline solid
crystals or chunks
반응 적합성
reagent type: catalyst
core: aluminum
pH
2.5-3.5 (20 °C)
SMILES string
Cl[Al](Cl)Cl.O.O.O.O.O.O
InChI
1S/Al.3ClH.6H2O/h;3*1H;6*1H2/q+3;;;;;;;;;/p-3
InChI key
JGDITNMASUZKPW-UHFFFAOYSA-K
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Aluminum chloride hexahydrate is a nonflammable compound widely employed as a catalyst in organic synthesis. In addition to being extremely soluble in water, it is also soluble in alcohol, ether, glycerol, propylene glycol, and acetonitrile. It has many uses, including the refinement of crude oil, the production of deodorants and antiperspirant products, the dyeing of fabrics, textile finishing.
애플리케이션
Aluminum chloride hexahydrate is used as a catalyst:
- In the synthesis of corresponding THP (tetrahydropyranylation) ether from alcohol or phenol, and 3,4-dihydro-2H-pyran (DHP).
- In the direct esterification of levulinic acid and ethanol to produce ethyl levulinate.
- In the synthesis of gem-dihydroperoxides from ketones and aldehydes.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT RE 1
표적 기관
Lungs
보충제 위험성
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
[Axillary hyperhydrosis. Local treatment with 25% aluminum chloride hexahydrate in absolute alcohol].
F Brandrup et al.
Ugeskrift for laeger, 140(35), 2106-2109 (1978-08-28)
Aluminium (III) chloride hexahydrate: an efficient and versatile reagent in organic synthesis.
Sarma SD, et al.
ARKIVOC (Gainesville, FL, United States), 1, 243-263 (2013)
Ingrid Siemund et al.
Contact dermatitis, 77(5), 288-296 (2017-07-12)
Contact allergy to aluminium has been reported more frequently in recent years. It has been pointed out that positive patch test reactions to aluminium may not be reproducible on retesting. To investigate possible variations in patch test reactivity to aluminium
Mark Baranov et al.
Inorganic chemistry, 58(13), 8877-8883 (2019-06-30)
While sophisticated computational methods can predict 31P NMR spectra of phosphorus atoms encapsulated within Keggin-derived heteropoly tungstate and molybdate cluster anions, calculated and experimental chemical shift values typically deviate considerably from one another. Motivated by the observation that experimentally determined
Solvent-free tetrahydropyranylation (THP) of alcohols and phenols and their regeneration by catalytic aluminum chloride hexahydrate.
Namboodiri VV and Varma RS.
Tetrahedron Letters, 43(7), 1143-1146 (2002)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.