모든 사진(4)
About This Item
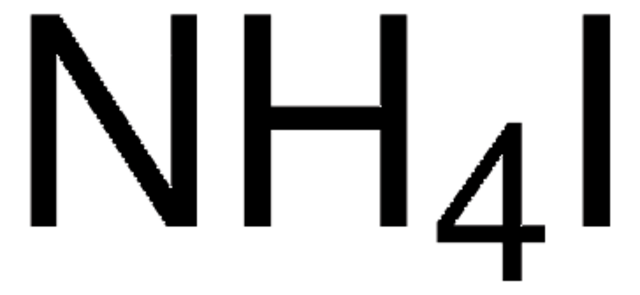
Linear Formula:
NH4I
CAS Number:
Molecular Weight:
144.94
EC Number:
MDL number:
UNSPSC 코드:
12352300
eCl@ss:
38050607
PubChem Substance ID:
분석:
≥99%
Grade:
ACS reagent
양식:
powder or crystals
추천 제품
Grade
ACS reagent
Quality Level
vapor pressure
1 mmHg ( 210.9 °C)
분석
≥99%
양식
powder or crystals
환경친화적 대안 제품 특성
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
불순물
≤0.005% insolubles
무기 잔류물
≤0.05%
pH
4.5-6.5 (25 °C, 50 g/L)
음이온 미량물
Cl- and Br-: ≤0.005%
phosphate (PO43-): ≤0.001%
sulfate (SO42-): ≤0.05%
양이온 미량물
Ba: ≤0.002%
Fe: ≤5 ppm
heavy metals (as Pb): ≤0.001%
환경친화적 대안 카테고리
SMILES string
N.I
InChI
1S/HI.H3N/h1H;1H3
InChI key
UKFWSNCTAHXBQN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is used as a ntrogen source for syntheis of many heterocyclic compounds and is also used as a catalyst or redox catalyst in some reactions. Click here for more information.
The high yield and regioselective conversion of an unactivated aziridine to an oxazolidinone using carbon dioxide with ammonium iodide as the catalyst
The high yield and regioselective conversion of an unactivated aziridine to an oxazolidinone using carbon dioxide with ammonium iodide as the catalyst
애플리케이션
Catalyst for mild, efficient, and greener dethioacetalization.
Catalyst for mild, efficient, and greener dethioacetalization.
Chemoselective Bromination of Active Methylene and Methyne Compounds by Potassium Bromide, Hydrochloric Acid and Hydrogen Peroxide
Chemoselective Bromination of Active Methylene and Methyne Compounds by Potassium Bromide, Hydrochloric Acid and Hydrogen Peroxide
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
STOT RE 1 Oral
표적 기관
Thyroid
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Jinho Kim et al.
Organic letters, 14(15), 3924-3927 (2012-07-17)
Copper-mediated regioselective cyanation of indoles and 2-phenylpyridines was developed by using ammonium iodide and DMF as the combined source of a cyano unit under "Pd-free" conditions. Mechanistic studies indicate that the reaction of indoles proceeds through a two-step sequence: electrophilic
Xiaofang Gao et al.
Chemical communications (Cambridge, England), 51(1), 210-212 (2014-11-20)
A novel ammonium iodide-induced sulfonylation of alkenes with DMSO and water toward the synthesis of vinyl methyl sulfones is described. The process proceeded smoothly under metal-free conditions with high stereoselectivity and good functional group tolerance. The reaction mechanism was revealed
Simple and regioselective oxyiodination of aromatic compounds with ammonium iodide and Oxone.
Mohan KVVK, et al.
Tetrahedron Letters, 45(43), 8015-8018 (2004)
Low-Frequency Vibrations in Ammonium Iodide and Ammonium Bromide.
Durig JR and Antion DJ.
J. Chem. Phys. , 51(9), 3639-3647 (1969)
Jinho Kim et al.
Journal of the American Chemical Society, 134(5), 2528-2531 (2012-01-28)
The cyanation of aromatic boronic acids, boronate esters, and borate salts was developed under copper-mediated oxidative conditions using ammonium iodide and DMF as the source of nitrogen and carbon atom of the cyano unit, respectively. The procedure was successfully extended
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.