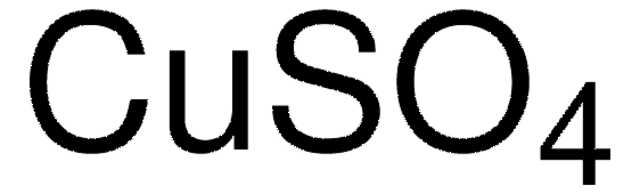12852
Copper(II) sulfate
puriss., meets analytical specification of Ph. Eur., BP, USP, anhydrous, 99-100.5% (based on anhydrous substance)
동의어(들):
Cupric sulfate
About This Item
추천 제품
vapor pressure
7.3 mmHg ( 25 °C)
Quality Level
grade
puriss.
분석
99-100.5% (based on anhydrous substance)
형태
solid
품질
meets analytical specification of Ph. Eur., BP, USP
불순물
residual solvents, complies
손실
≤1% loss on drying, 250 °C
pH
3.5-4.5 (20 °C, 50 g/L)
mp
200 °C (dec.) (lit.)
density
3.603 g/mL at 25 °C (lit.)
음이온 미량물
chloride (Cl-): ≤100 mg/kg
양이온 미량물
Ca: ≤50 mg/kg
Fe: ≤30 mg/kg
K: ≤100 mg/kg
Mg: ≤100 mg/kg
Na: ≤200 mg/kg
Ni: ≤50 mg/kg
Pb: ≤80 mg/kg
SMILES string
[Cu++].[O-]S([O-])(=O)=O
InChI
1S/Cu.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2
InChI key
ARUVKPQLZAKDPS-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
It can find applications in agriculture as a nutrient for plants. It can also serve as a precursor in the production of foliar bactericides and fungicides.
애플리케이션
- As a catalyst for the acetylation of alcohols and phenols under solvent-free conditions.
- To compose the electrolyte for the electrodeposition of Cu-Zn-Sn precursors, required for the preparation of Cu2ZnSnS4 (CZTS) thin films.
- As a Lewis acid catalyst for the dehydration of alcohols.5
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.