추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. P2660000
traceable to USP 1551503
API family
povidone
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
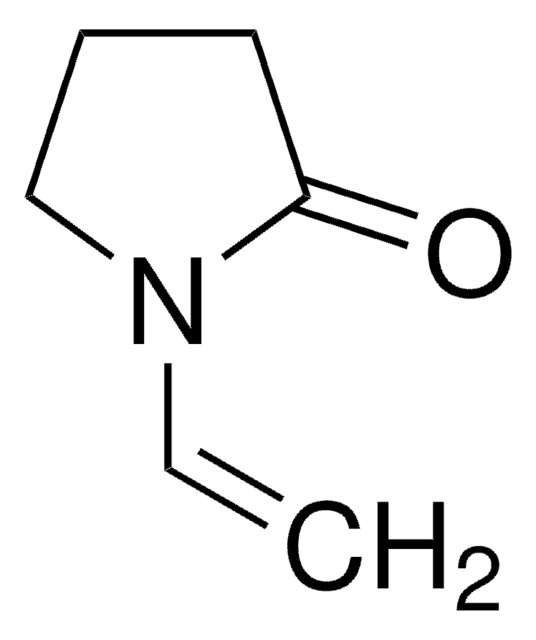
SMILES string
C=CN1CCCC1=O
InChI
1S/C6H9NO/c1-2-7-5-3-4-6(7)8/h2H,1,3-5H2
InChI key
WHNWPMSKXPGLAX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
Povidone may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
기타 정보
Polyvinylpyrrolidone is a component of Denhardt′s Solution and is included at a concentration of 1% (w/v) in the standard 50X stock solution.
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
각주
To see an example of a Certificate of Analysis for this material enter LRAC3638 in the slot below. This is an example certificate only and may not be the lot that you receive.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Determination of bile acids in pharmaceutical formulations using micellar electrokinetic chromatography
Rodriguez VG, et al.
Journal of Pharmaceutical and Biomedical Analysis, 23(2-3), 375-381 (2000)
Ultra rapid liquid chromatography as second dimension in a comprehensive two-dimensional method for the screening of pharmaceutical samples in stability and stress studies
Huidobro AL, et al.
Journal of Chromatography A, 1190(1-2), 182-190 (2008)
The simultaneous separation and determination of five quinolone antibotics using isocratic reversed-phase HPLC: Application to stability studies on an ofloxacin tablet formulation
Shervington LA, et al.
Journal of Pharmaceutical and Biomedical Analysis, 39(3-4), 769-775 (2005)
Detection and quantification of low-molecular-weight aldehydes in pharmaceutical excipients by headspace gas chromatography
Li Z, et al.
Journal of Chromatography A, 1104(1-2), 1-10 (2006)
Doris Bach et al.
Journal of photochemistry and photobiology. B, Biology, 120, 74-81 (2013-03-08)
Photodynamic therapy (PDT) is a local tumour treatment accepted for a number of indications. PDT operates via the cellular stress response through the production of reactive oxygen species and subsequent cellular damage, resulting in cell death. Although PDT-induced signalling and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.