P3750000
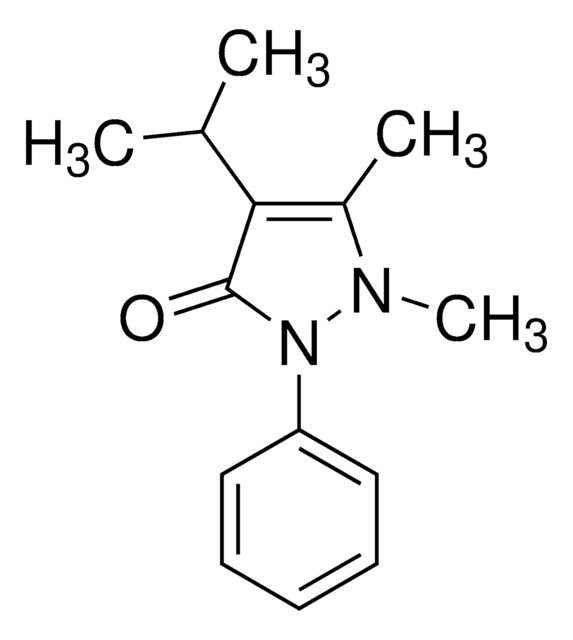
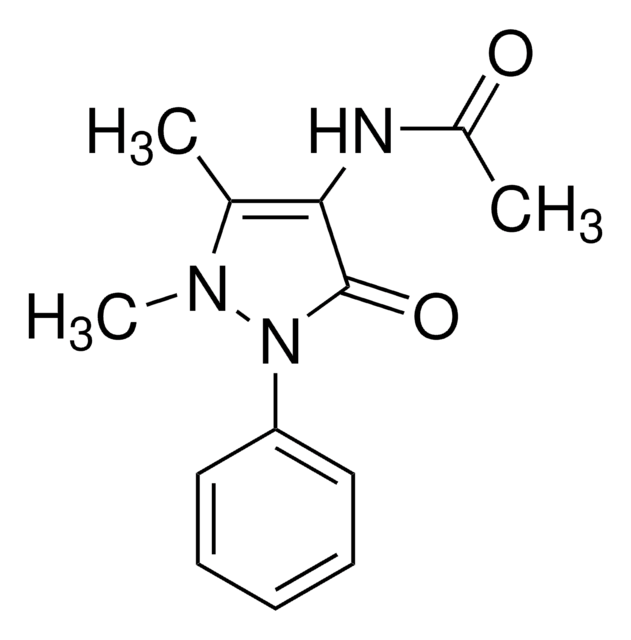
Propyphenazone
European Pharmacopoeia (EP) Reference Standard
동의어(들):
4-Isopropylantipyrine, 1,2-Dihydro-1,5-dimethyl-4-(1-methylethyl)-2-phenyl-3H-pyrazol-3-one, 1,2-Dihydro-4-isopropyl-1,5-dimethyl-2-phenyl-3H-pyrazol-3-one, Isopropylphenazone, Propyphenazone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C14H18N2O
CAS Number:
Molecular Weight:
230.31
Beilstein:
204533
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
propyphenazone
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
O=C1C(C(C)C)=C(C)N(C)N1C2=CC=CC=C2
InChI
1S/C14H18N2O/c1-10(2)13-11(3)15(4)16(14(13)17)12-8-6-5-7-9-12/h5-10H,1-4H3
InChI key
PXWLVJLKJGVOKE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Propyphenazone EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Deniz Emre et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 847(2), 126-132 (2006-10-19)
A new micellar electrokinetic capillary chromatographic method has been developed to analyze the pharmaceutical preparations containing ternary combination of paracetamol (PAR), caffeine (CAF) and propyphenazone (PRO). Best results were obtained by using 20mM pH 9.0 borate buffer containing 30mM sodiumdodecylsulphate
Andreas Lemmerer et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(48), 13445-13460 (2011-11-15)
A co-crystal of two polymorphic active pharmaceutical ingredients (APIs), first reported and patented in 1937, has been prepared and thoroughly characterised, including crystal structure analysis. The existence of four crystal forms of one of the APIs, the sedative and hypnotic
Sebastian Zühlke et al.
Journal of chromatography. A, 1050(2), 201-209 (2004-10-29)
A new analytical method applying in situ derivatization was developed to enable the extraction of polar drug metabolites from water samples by solid-phase extraction (SPE). An additional derivatization by silylation was used to enhance the sensitivity of analyte detection by
Acute inferior myocardial infarction with low atrial rhythm due to propyphenazone: Kounis syndrome.
Ahmet Akyel et al.
International journal of cardiology, 148(3), 352-353 (2010-06-15)
Rosario Rodil et al.
Water research, 46(7), 2457-2468 (2012-03-03)
Chlorination is one of the most popular disinfection steps for water treatment in Europe. However, chlorine can react with pharmaceuticals and other micropollutants leading to either their elimination or by-products being formed. These by-products are frequently not identified and therefore
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.