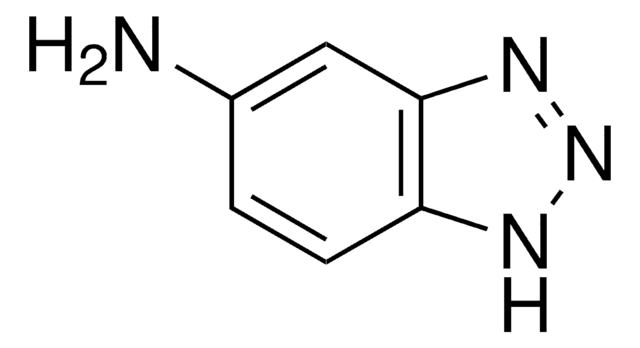
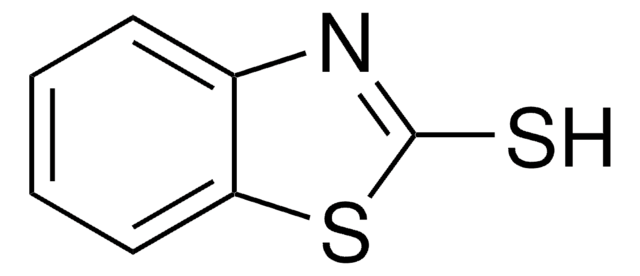
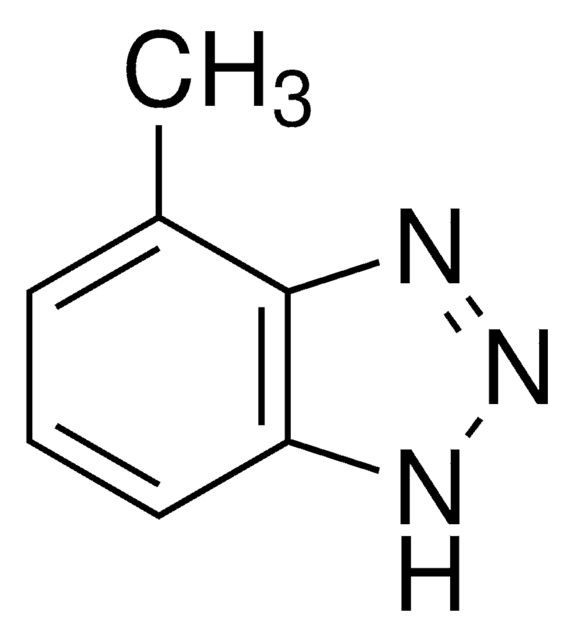
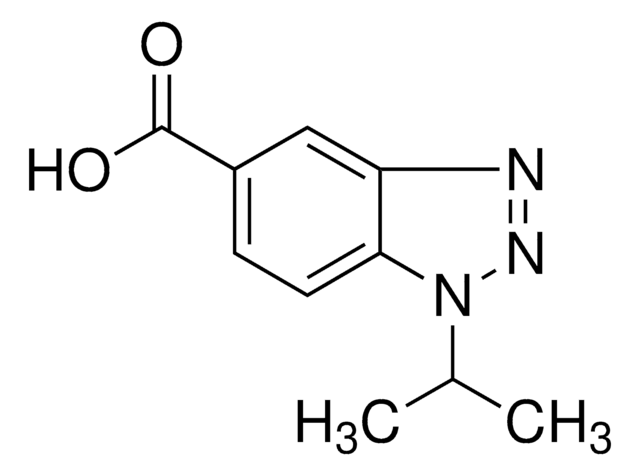
추천 제품
vapor density
4.1 (vs air)
Quality Level
vapor pressure
0.04 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
99%
형태
powder
mp
97-99 °C (lit.)
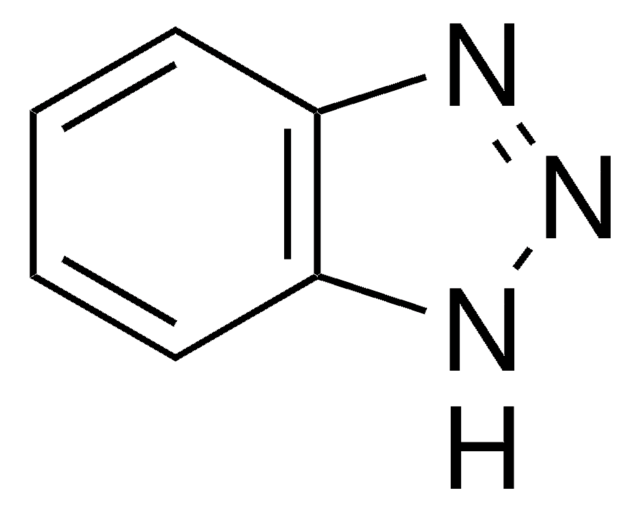
SMILES string
c1ccc2[nH]nnc2c1
InChI
1S/C6H5N3/c1-2-4-6-5(3-1)7-9-8-6/h1-4H,(H,7,8,9)
InChI key
QRUDEWIWKLJBPS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Benzotriazole is used as a synthetic auxiliary for the preparation of organic derivatives and as a corrosion inhibitors for Cu and its alloys.
애플리케이션
Benzotriazole can be used as a reactant to synthesize:
- β-Aminocarbonyl compounds via Mannich reaction of secondary amines and aldehydes in the presence of p-toluenesulfonic acid as a catalyst.
- Acylbenzotriazoles via thionyl chloride catalyzed reaction with nitrobenzoic acids.
- 1-(2-Pyridyl)benzotriazole by reacting with 2-bromopyridine in the presence of toluene as a solvent.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
338.0 °F - closed cup
Flash Point (°C)
170 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Synthesis, Characterization and Energetic Properties of 1, 3, 4-Oxadiazoles
Wang Z, et al.
European Journal of Organic Chemistry, 2015, 5183-5188 (2015)
Derivatization of 1-phenyl substituted 4-amino-2-benzazepin-3-ones: evaluation of Pd-catalyzed coupling reactions
Ballet S, et al.
Tetrahedron, 63, 3718-3727 (2007)
Martin Krug et al.
ChemMedChem, 6(1), 63-72 (2010-12-09)
Within the last decade, interest in the development of new anticancer drugs increased mainly from emerging resistance against established drugs, which were found to be limited by the multidrug resistance (MDR) phenomenon. Several anticancer targets have been investigated for the
Monica Tonelli et al.
Materials (Basel, Switzerland), 13(14) (2020-07-19)
The ingress of water, as a vehicle for many harmful substances, is the main cause of all the major physical and chemical degradation processes affecting concrete buildings. To prevent damage and protect concrete surfaces, coatings are generally used. Cement-based coatings
Jiufeng Li et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 1070, 70-75 (2017-11-02)
Benzotriazole (BTR) and benzothiazole (BTH) derivatives are extensively applied in industrial processes and consumer products, and are thus frequently detected in the environmental matrices. Due to their potential estrogenic effects reported in animal studies, the assessment of human exposure to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.