923133
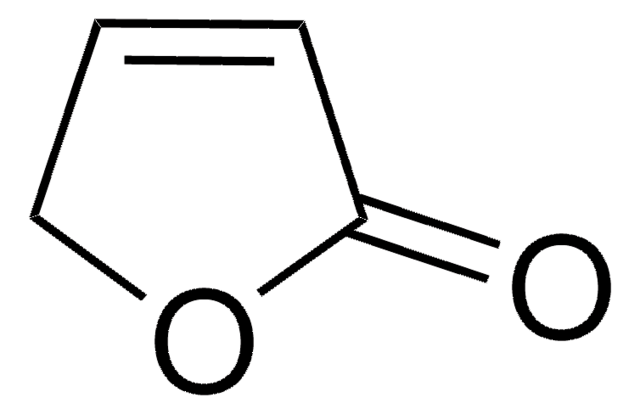
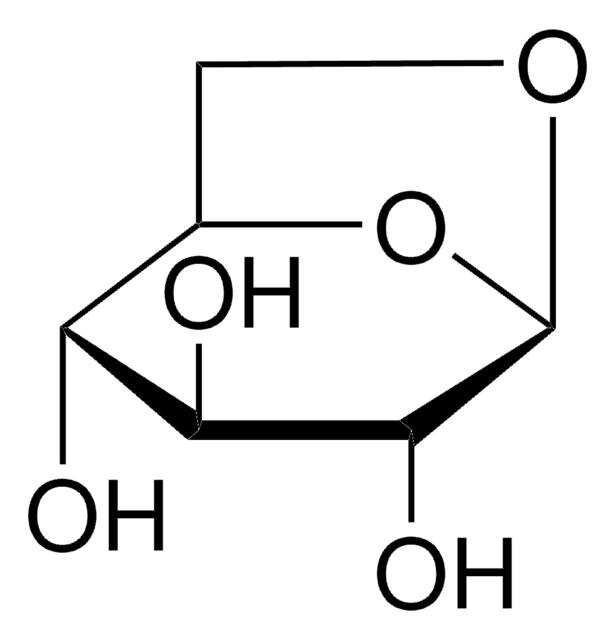
Levoglucosenone
≥95%
동의어(들):
(-)-Levoglucosenone, LGO, (1S,5R)-6,8-dioxabicyclo[3.2.1]oct-2-en-4-one, 1,6-anhydro-3,4-dideoxyhex-3-enopyran-2-ulose, 6,8-Dioxabicyclo[3.2.1]oct-2-en-4-one
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C6H6O3
CAS Number:
Molecular Weight:
126.11
MDL number:
UNSPSC 코드:
12352201
NACRES:
NA.22
추천 제품
Quality Level
분석
≥95%
양식
liquid
환경친화적 대안 제품 특성
Safer Solvents and Auxiliaries
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n/D 1.50625
density
1.32 g/mL
작용기
ether
ketone
환경친화적 대안 카테고리
, Aligned
저장 온도
2-8°C
SMILES string
[H][C@]1(C=CC2=O)CO[C@]2([H])O1
InChI
1S/C6H6O3/c7-5-2-1-4-3-8-6(5)9-4/h1-2,4,6H,3H2/t4-,6+/m0/s1
InChI key
HITOXZPZGPXYHY-UJURSFKZSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
We are committed to bringing you greener alternative products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is biorenewable and thus aligns with ″Safer Solvents and Auxiliaries″ and ″Use of Renewable Feedstocks″. Click here for more information.
Levoglucosenone undergoes Michael-addition reaction with diethyl malonate, ethyl cyanoacetate, 2-nitropropane and 2-methylcyclohexanone in the presence of various catalysts to afford 4-substituted levoglucosenone derivatives having the D-erythro configuration.
Levoglucosenone undergoes Michael-addition reaction with diethyl malonate, ethyl cyanoacetate, 2-nitropropane and 2-methylcyclohexanone in the presence of various catalysts to afford 4-substituted levoglucosenone derivatives having the D-erythro configuration.
애플리케이션
Levoglucosenone may be used in the synthesis of the following:
- Various deoxy, keto and branched-chain sugars
- 4-thio and 4-substituted sugar derivatives
- Natural stereoisomer of serricornin
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Levoglucosenone and Its New Applications: Valorization of Cellulose Residues.
Comba M B, et al.
European Journal of Organic Chemistry, 590-604 (2018)
Jonas Kühlborn et al.
Natural product reports, 37(3), 380-424 (2019-10-19)
Covering: up to mid-2019 This review highlights the utilization of biomass-derived building blocks in the total synthesis of natural products. An overview over several renewable feedstock classes, namely wood/lignin, cellulose, chitin and chitosan, fats and oils, as well as terpenes
Recent Applications of Levoglucosenone as Chiral Synthon.
Sarotti A M, et al.
Current Organic Synthesis, 9(4), 439-459 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![(1S,5R)-6,8-Dioxabicyclo[3.2.1]oct-2-en-4-one](/deepweb/assets/sigmaaldrich/product/structures/338/530/ef82365a-1396-4598-9576-3680445a1c50/640/ef82365a-1396-4598-9576-3680445a1c50.png)