추천 제품
Grade
for GC derivatization
Quality Level
분석
≥99.0% (GC)
≥99.0%
형태
crystals
품질
LiChropur™
반응 적합성
reagent type: derivatization reagent
reaction type: Acylations
기술
gas chromatography (GC): suitable
bp
135 °C/18 mmHg (lit.)
mp
48-51 °C (lit.)
49-50 °C
저장 온도
2-8°C
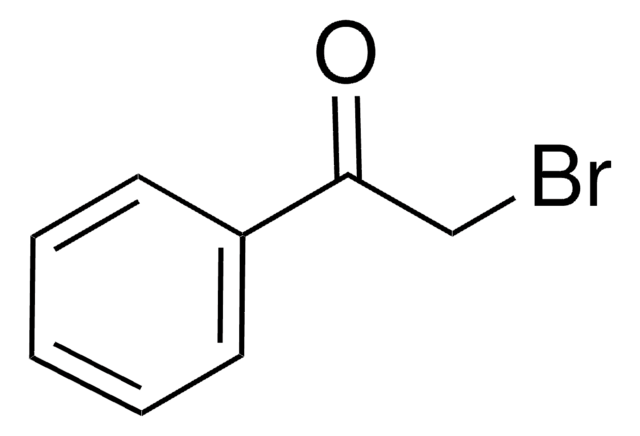
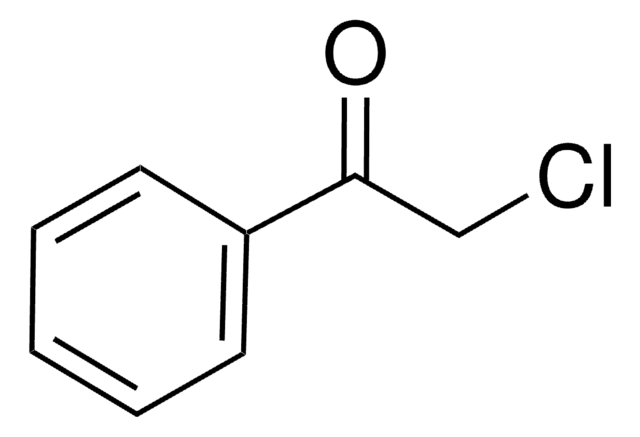
SMILES string
BrCC(=O)c1ccccc1
InChI
1S/C8H7BrO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
InChI key
LIGACIXOYTUXAW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Bromoacetophenone is a commonly used derivatization agent for fatty acid detection in biological samples.
애플리케이션
Preparation of crystalline esters from acids
법적 정보
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
High-performance liquid chromatography of fatty acids in biological samples.
Lima ES and Abdalla DSP
Analytica Chimica Acta, 465(1-2), 81-91 (2002)
P A Wender et al.
Organic letters, 1(13), 2117-2120 (2000-06-03)
[formula: see text] 4'-Bromoacetophenone derivatives which upon excitation can generate monophenyl radicals capable of hydrogen atom abstraction were investigated as photoinducible DNA cleaving agents. Pyrrolecarboxamide-conjugated 4'-bromoacetophenones were synthesized, and their DNA cleaving activities and sequence selectivities were determined.
Gulnur Arabaci et al.
Bioorganic & medicinal chemistry letters, 12(21), 3047-3050 (2002-10-10)
A series of alpha-haloacetophenone derivatives was tested for inhibition of protein tyrosine phosphatases SHP-1 and PTP1B. The results show that the bromides are much more potent than the corresponding chlorides, whereas the phenyl ring is remarkably tolerant to modifications. Derivatization
T Endoh et al.
Carcinogenesis, 17(3), 467-475 (1996-03-01)
Effects of inhibitors of arachidonic acid (AA) metabolism on the development of fatty liver, cirrhosis, glutathione-S-transferase placental form (GST-P)-positive nodules and the generation of 8-hydroxydeoxyguanosine (8-OHdG) and thiobarbituric acid-reactive substances (TBARS), caused by a choline-deficient, L-amino acid-defined (CDAA) diet, were
Mostafa A Hussein et al.
Acta pharmaceutica (Zagreb, Croatia), 59(4), 365-382 (2009-11-19)
5-Acyl-8-hydroxyquinoline-2-(3'-substituted-4'-aryl-2,3-dihydrothiazol-2'-ylidene)hydrazones, 5a-e to 10a-c, were prepared by the reaction of appropriate 5-acyl-8-hydroxyquinoline-4-substituted thiosemicarbazones 3a-e and phenacyl bromides 4a-e. Structures of the new compounds were verified on the basis of spectral and elemental analyses. Twenty-eight new compounds were tested for their
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.