추천 제품
Quality Level
분석
≥70.0% (HPCE)
양식
powder
mp
135-136 °C (lit.)
solubility
DMF: soluble
DMSO: soluble
acetonitrile: soluble
methanol: soluble
형광
λex 380 nm; λem 461 nm in methanol
λex 390 nm; λem 478 nm in 0.1 M phosphate pH 7.5 (after derivatization with glutathione)
적합성
suitable for fluorescence
SMILES string
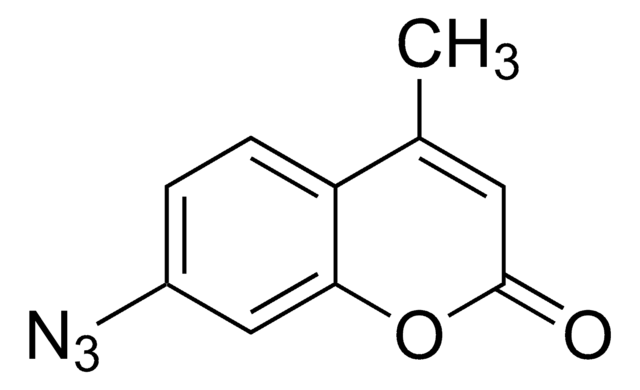
CC1=C(C)C(=O)N2N1C(CCl)=C(C)C2=O
InChI
1S/C10H11ClN2O2/c1-5-7(3)12-8(4-11)6(2)10(15)13(12)9(5)14/h4H2,1-3H3
InChI key
SUIPVTCEECPFIB-UHFFFAOYSA-N
일반 설명
Monochlorobimane is a glutathione (GSH) fluorescent cell-permeable probe. When incubated with the test cell culture, it readily enters the cells and forms a fluorescent complex. The Monochlorobimane-GSH reaction is catalyzed by glutathione-S-transferase, which is detected fluorometrically.
Monochlorobimane, also known as mBCl, is a non-fluorescent compound that forms a fluorescent complex upon reaction. The fluorescence is detected at 394/490nm.
Monochlorobimane, also known as mBCl, is a non-fluorescent compound that forms a fluorescent complex upon reaction. The fluorescence is detected at 394/490nm.
애플리케이션
Monochlorobimane is used as a fluorescent agent in fluorometric glutathione assays. It is used to detect the principal intracellular low-molecular-weight thiols, which play a pivotal role in the defense mechanism.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Jordi Sebastià et al.
Cytometry. Part A : the journal of the International Society for Analytical Cytology, 51(1), 16-25 (2002-12-25)
Reduced glutathione (GSH) protects cells against oxidative injury and maintains a range of vital functions. To study GSH content in human neuronal cell cultures, thiol-sensitive fluorescent techniques requiring a small number of cells may be of great value, but their
Natascha Rauch et al.
Insect biochemistry and molecular biology, 34(4), 321-329 (2004-03-26)
Glutathione S-transferases (GST) catalyzing the conjugation of reduced glutathione to a vast range of xenobiotics including insecticides were characterized in the whitefly Bemisia tabaci. GST activities were determined in susceptible and resistant strains of B. tabaci towards artificial substrates, i.e.
S Nair et al.
Cytometry, 12(4), 336-342 (1991-01-01)
We have used an enzymatic (spectro-photometric) and a flow cytometric (GSH-MBCL) method to compare the glutathione (GSH) content of doxorubicin sensitive (P388) and resistant (P388/R-84) murine leukemic and human lung cancer cells. The flow cytometric analysis revealed that GSH-MBCL conjugate
Formation and rapid export of the monochlorobimane-glutathione conjugate in cultured rat astrocytes.
Jens Waak et al.
Neurochemical research, 31(12), 1409-1416 (2006-11-08)
Monochlorobimane (MCB) is often used to visualize glutathione (GSH) levels in cultured cells, since it is quickly converted to a fluorescent GSH conjugate (GS-MCB). To test for consequences of MCB application on the GSH metabolism of astrocytes, we have studied
Madushi Raththagala et al.
Analytical chemistry, 78(24), 8556-8560 (2006-12-15)
A method for the quantitative determination of the antioxidant form of glutathione (GSH) in red blood cells (RBCs) is described that does not require separation of the analyte of interest from the complex cellular matrix. The measurement portion of the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.