추천 제품
애플리케이션
- 1,5-Diazabicyclo[4.3.0]non-5-ene (DBN) is an amidine base commonly used in the base-mediated eliminations, condensations, esterifications, isomerizations, carboxylations and carbonylations.
- It is used in the preparation of supertetrahedral chalcogenide clusters and single crystals of polymer-chalcogenide composites.
- It also acts as a catalyst for the regioselective Friedel-Crafts C-acylation of pyrroles.
기타 정보
Amidine base used for dehydrohalogenation reactions to olefins
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
201.2 °F - closed cup
Flash Point (°C)
94 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
Nucleophilicities and carbon basicities of DBU and DBN.
Baidya, M and Mayr, Herbert
Chemical Communications (Cambridge, England), 1792-1794 (2008)
Threading chalcogenide layers with polymer chains.
Xiong, Wei-Wei et al.
Angewandte Chemie (International Edition in English), 127(2), 556-560 (2015)
Superbase route to supertetrahedral chalcogenide clusters.
Wu, Tao et al.
Journal of the American Chemical Society, 134(8), 3619-3622 (2012)
1, 5-Diazabicyclo [4.3. 0] non-5-ene.
Savoca, Ann C and Urgaonkar, Sameer
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Andrzej E Wróblewski et al.
The Journal of organic chemistry, 67(2), 420-425 (2002-01-19)
Although P(CH(3)NCH(2)CH(2))(3)N (1) was found to be less effective than 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in the removal of hydrogen bromide from vitamin A intermediates 13-cis-10-bromo-9,10-dihydroretinyl acetates (6) and 14-bromo-9,14-dihydroretinyl acetate (11) when the reaction was carried out in refluxing
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![1,5-Diazabicyclo[4.3.0]non-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/400/401/859b2474-712b-4448-b231-74d0bc3203f1/640/859b2474-712b-4448-b231-74d0bc3203f1.png)
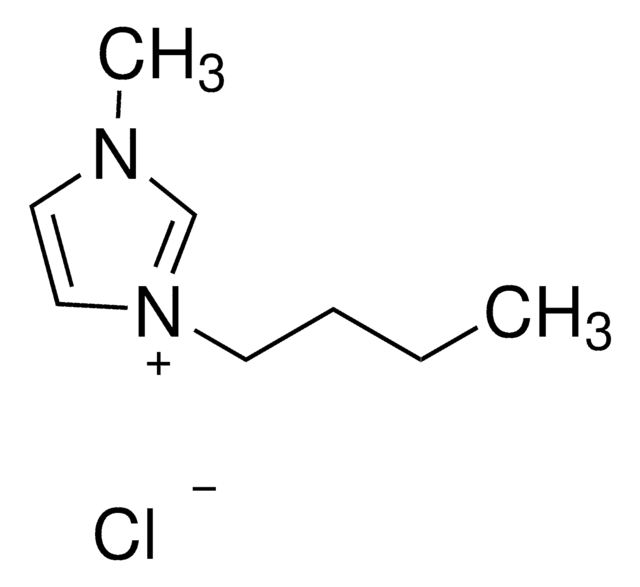
![1,8-Diazabicyclo[5.4.0]undec-7-ene puriss., ≥99.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)


