추천 제품
Grade
for ion-selective electrodes
Quality Level
제품 라인
Selectophore™
분석
≥97.0% (TLC)
저장 온도
2-8°C
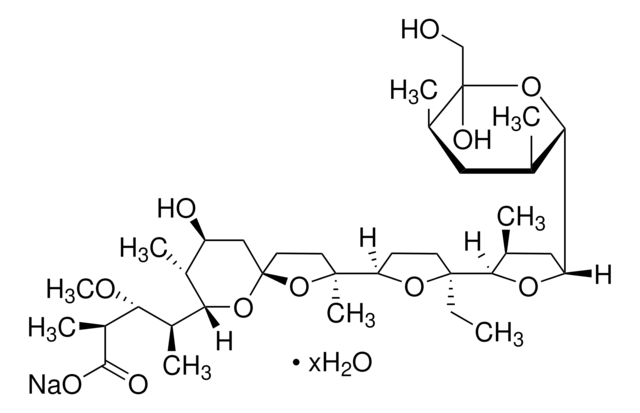
SMILES string
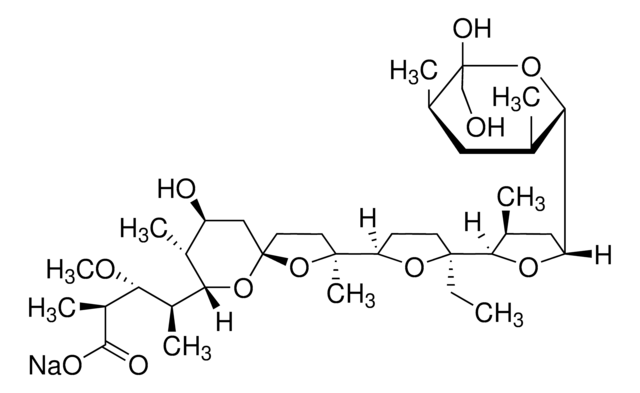
CC[C@]1(CCC(O1)[C@]2(C)CC[C@]3(C[C@H](O)[C@@H](C)C(O3)[C@@H](C)[C@@H](OC)[C@H](C)C(=O)OC)O2)C4OC(C[C@@H]4C)C5O[C@@](O)(CO)[C@H](C)C[C@@H]5C
InChI
1S/C37H64O11/c1-11-35(32-21(3)17-27(44-32)29-20(2)16-22(4)37(41,19-38)47-29)13-12-28(45-35)34(8)14-15-36(48-34)18-26(39)23(5)31(46-36)24(6)30(42-9)25(7)33(40)43-10/h20-32,38-39,41H,11-19H2,1-10H3/t20-,21+,22+,23+,24+,25+,26-,27-,28+,29-,30-,31-,32-,34+,35-,36+,37-/m0/s1
InChI key
PFRZSHIENRKVSE-RJTHVKINSA-N
일반 설명
애플리케이션
- The selectivity of membrane ion-selective electrodes: This research explores the use of temperature variations to adjust the selectivity of ion-selective electrodes, utilizing Monensin methyl ester as a key component for sodium ion detection (Zahran et al., 2010).
- Spectroscopic and semiempirical studies of a proton channel formed by the methyl ester of monensin A.: This paper presents a detailed analysis of the proton channel properties of Monensin methyl ester through spectroscopic and computational methods, highlighting its potential in analytical chemistry applications (Huczyński et al., 2006).
- Ion chromatography detector based on solid-state ion-selective electrode array.: The development of an ion chromatography detector employing solid-state ion-selective electrodes, with Monensin methyl ester playing a crucial role in sodium ion detection, is detailed in this study (Lee et al., 2000).
포장
법적 정보
관련 제품
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.