추천 제품
Quality Level
제품 라인
Novabiochem®
분석
≥98% (TLC)
≥99.0% (HPLC)
형태
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
제조업체/상표
Novabiochem®
mp
>65 °C
응용 분야
peptide synthesis
작용기
Fmoc
저장 온도
-10 to -25°C
InChI
1S/C34H40N4O7S/c1-19-20(2)30(21(3)26-17-34(4,5)45-29(19)26)46(42,43)38-32(35)36-16-10-15-28(31(39)40)37-33(41)44-18-27-24-13-8-6-11-22(24)23-12-7-9-14-25(23)27/h6-9,11-14,27-28H,10,15-18H2,1-5H3,(H,37,41)(H,39,40)(H3,35,36,38)/t28-/m0/s1
InChI key
HNICLNKVURBTKV-NDEPHWFRSA-N
일반 설명
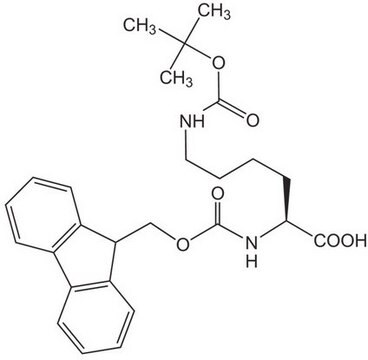
High purity Fmoc-protected amino acid for research and process production of peptides, with very low levels of dipeptide, free-amino acids and acetic acid impurities.
The standard derivative for the introduction of Arg in Fmoc SPPS [1,2]. The Pbf side-chain protecting group is removed with TFA approximately 1-2 times faster than Pmc.In the preparation of peptides containing both Arg and Trp, it is recommended that this derivative is used in conjunction with Fmoc-Trp(Boc)-OH (852050).
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] L. A. Carpino, et al. (1993) Tetrahedron Lett., 34, 7829.
[2] C. G. Fields, et al. (1993) Tetrahedron Lett., 34, 6661.
The standard derivative for the introduction of Arg in Fmoc SPPS [1,2]. The Pbf side-chain protecting group is removed with TFA approximately 1-2 times faster than Pmc.In the preparation of peptides containing both Arg and Trp, it is recommended that this derivative is used in conjunction with Fmoc-Trp(Boc)-OH (852050).
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] L. A. Carpino, et al. (1993) Tetrahedron Lett., 34, 7829.
[2] C. G. Fields, et al. (1993) Tetrahedron Lett., 34, 6661.
애플리케이션
- Syntheses of cyanophycin segments for investigations of cell-penetration: Describes the synthesis process and overall yields for steps from Fmoc-Arg(Pbf)-OH to Adp building blocks. (M Grogg, D Hilvert, D Seebach, 2019).
- Revisiting NO2 as Protecting Group of Arginine in Solid-Phase Peptide Synthesis: Compares the stability of Fmoc-Arg(Boc) 2 -OH with Fmoc-Arg(Pbf)-OH in solvents over time. (M Alhassan, A Kumar, J Lopez, F Albericio, 2020).
- Gene delivery of PAMAM dendrimer conjugated with the nuclear localization signal peptide originated from fibroblast growth factor 3: Discusses a conjugation reaction involving Fmoc-Arg(pbf)-OH in gene delivery systems. (J Lee, J Jung, YJ Kim, E Lee, JS Choi, 2014).
결합
Replaces: 04-12-1145
분석 메모
Colour (visual): white to off white
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.8 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Arg(Pbf)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Arg(Pbf)-Arg(Pbf)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Arg-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (HPLC): ≤ 0.2 %
Purity (TLC(011C)): ≥ 98 %
Purity (TLC(157B)): ≥ 98 %
Solubility (12,5 mmol in 25 ml DMF): clearly soluble
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 1.0 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.8 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Arg(Pbf)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Arg(Pbf)-Arg(Pbf)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Arg-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (HPLC): ≤ 0.2 %
Purity (TLC(011C)): ≥ 98 %
Purity (TLC(157B)): ≥ 98 %
Solubility (12,5 mmol in 25 ml DMF): clearly soluble
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 1.0 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
법적 정보
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Yun Song et al.
Cell reports, 30(8), 2699-2711 (2020-02-27)
The transcriptional corepressor complex CoREST is one of seven histone deacetylase complexes that regulate the genome through controlling chromatin acetylation. The CoREST complex is unique in containing both histone demethylase and deacetylase enzymes, LSD1 and HDAC1, held together by the
관련 콘텐츠
Purer Fmocs Means Purer Peptides
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.