506172
p38 MAP Kinase Inhibitor X, BIRB 796
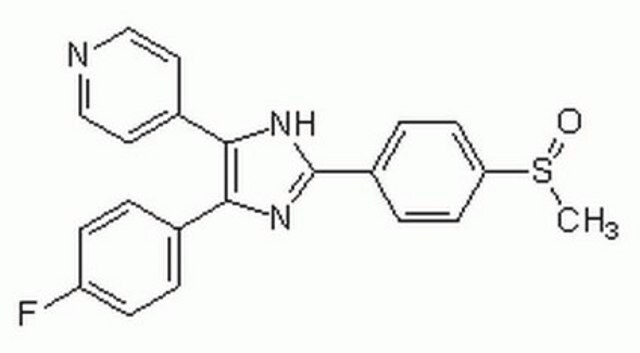
The p38 MAP Kinase Inhibitor X, BIRB 796, also referenced under CAS 285983-48-4, controls the biological activity of p38 MAP Kinase. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
동의어(들):
p38 MAP Kinase Inhibitor X, BIRB 796, Doramapimod, BIRB796, 1-(5- tert-Butyl-2- p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea, JNK Inhibitor XVII, Doramapimod, BIRB796, 1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea, JNK Inhibitor XVII
About This Item
추천 제품
Quality Level
분석
≥97% (HPLC)
형태
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
protect from light
색상
white
solubility
DMSO: 50 mg/mL, clear, colorless
배송 상태
ambient
저장 온도
2-8°C
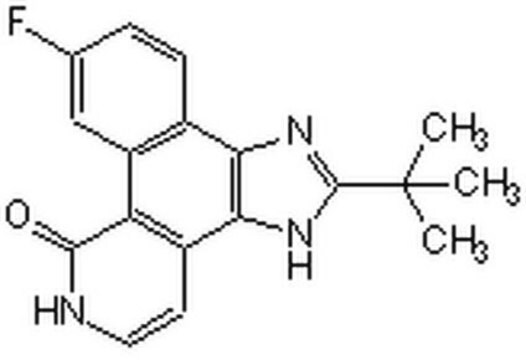
SMILES string
CC1=CC=C(N2C(NC(NC3=CC=C(OCCN4CCOCC4)C5=C3C=CC=C5)=O)=CC(C(C)(C)C)=N2)C=C1
InChI
1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37)
InChI key
MVCOAUNKQVWQHZ-UHFFFAOYSA-N
일반 설명
포장
경고
재구성
기타 정보
Regan, J., et al. 2003. J. Med. Chem.46, 4676.
Pargellis, C., et al. 2002. Nat. Struct. Biol.9, 268.
Regan, J., et al. 2002. J. Med. Chem.45, 2994.
법적 정보
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.