385585
17β-Hydroxysteroid Dehydrogenase Type-3 Inhibitor
The 17β-Hydroxysteroid Dehydrogenase Type-3 Inhibitor controls the biological activity of 17β-Hydroxysteroid Dehydrogenase Type-3.
동의어(들):
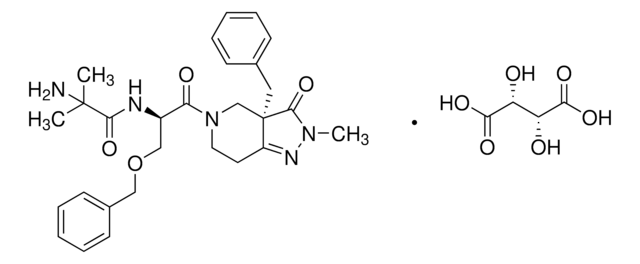
17β-Hydroxysteroid Dehydrogenase Type-3 Inhibitor, 17β-HSD3 Inhibitor, 5-(3-Bromo-4-hydroxybenzylidene)-3-(4-methoxyphenyl)-2-thioxothiazolidin-4-one
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C17H12BrNO3S2
CAS Number:
Molecular Weight:
422.32
MDL number:
UNSPSC 코드:
12352200
NACRES:
NA.77
추천 제품
Quality Level
분석
≥97% (HPLC)
양식
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
protect from light
색상
yellow
solubility
DMSO: 50 mg/mL, pink
배송 상태
ambient
저장 온도
2-8°C
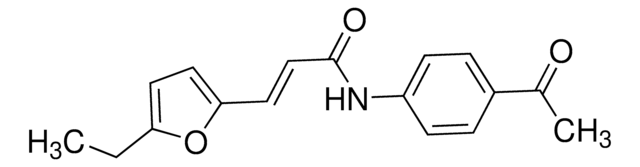
SMILES string
O=C(N(C(S/1)=S)C2=CC=C(C=C2)OC)C1=C\C3=CC=C(O)C(Br)=C3
InChI key
HEFKAUYVLSGPGC-DHDCSXOGSA-N
일반 설명
A cell-permeable benzylidine-thioxothiazolidinone compound that blocks the steroidogenesis of testosterone by directly inhibiting 17β-HSD3- (17β-hydroxysteroid dehydrogenase type 3) catalyzed, NADPH-dependent, reduction of Δ4-dione C17 ketone in a Δ4-dione- (4-androstene-3,17-dione) competitive, highly potent (IC50 = 0.6, 6.0, and 40 nM, respectively, against momoset, human, and mouse 17β-HSD3 activity in testes homogenate), and selective manner, displaying no activity against 17β-HSD1 and 17β-HSD2 activity. Poor pharmacokinetic properties limit its use to culture treatments and cell-free assays only.
A cell-permeable benzylidine-thioxothiazolidinone compound that blocks the steroidogenesis of the potent androgen testosterone by directly inhibiting 17β-HSD3- (17β-hydroxysteroid dehydrogenase type 3) catalyzed, NADPH-dependent, reduction of Δ4-dione C17 ketone in a Δ4-dione- (4-androstene-3,17-dione) competitive, highly potent (IC50 = 0.6, 6.0, and 40 nM, respectively, against momoset, human, and mouse 17β-HSD3 activity in testes homogenate; IC50 = 14 nM using 17β HSD3-transfected HeLa cells), and selective manner, displaying no activity against 17β-HSD1 and 17β-HSD2 activity in HeLa transfectants or AR- (andogen receptor), ERα- (estrogen receptor α), and GR- (glucocorticoid receptor) dependent transcription activities. Low aqueous solubility, lack of oral availability, and other poor pharmacokinetic properties limit its use to culture treatments and cell-free assays only.
포장
Packaged under inert gas
경고
Toxicity: Standard Handling (A)
재구성
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
기타 정보
Harada, K., et al. 2012. Bioorg. Med. Chem. Lett.22, 504.
법적 정보
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Yating Cheng et al.
Frontiers in pharmacology, 11, 637-637 (2020-05-28)
The 17β-hydroxysteroid dehydrogenase type 3 (17β-HSD3) enzyme is a potential therapeutic target for hormone-dependent prostate cancer, as it is the key enzyme in the last step of testosterone (T) biosynthesis. A curcumin analog, H10, was optimized for inhibiting T production
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.