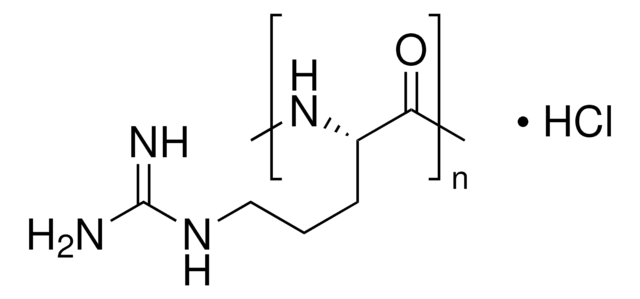
341006
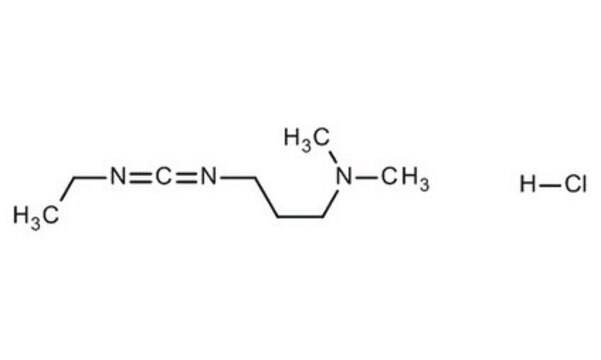
EDAC, Hydrochloride
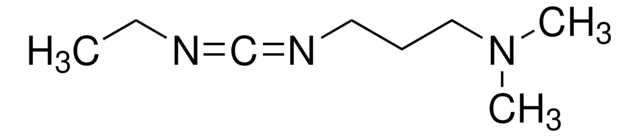
EDAC HCl is a water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis.
동의어(들):
EDAC, Hydrochloride, EDCI, 1-Ethyl-3-(3ʹ-dimethylaminopropyl)carbodiimide, HCl
About This Item
추천 제품
Quality Level
설명
RTECS - FF2200000
분석
≥98% (titration)
양식
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
desiccated
색상
white
solubility
aqueous buffer: 2-5 mg/mL
water: 2-5 mg/mL
배송 상태
ambient
저장 온도
−20°C
SMILES string
Cl.N(CCCN(CC)C#N)(C)C
InChI
1S/C8H17N3.ClH/c1-4-11(8-9)7-5-6-10(2)3;/h4-7H2,1-3H3;1H
InChI key
FDXPUDRRFDHONO-UHFFFAOYSA-N
일반 설명
포장
경고
제조 메모
재구성
기타 정보
Richardson, A., et al. 1992. Biochem. Pharmacol. 43, 1415.
Taniuchi, M., et al. 1986. Proc. Natl. Acad. Sci. USA83, 1950.
Chase, J.W., et al. 1983. Proc. Natl. Acad. Sci. USA80, 5480.
Williams, A., et al. 1981. J. Am. Chem. Soc.103, 7090.
Yamada, H., et al. 1981. Biochemistry20, 4836.
Thomas, J.O., et al. 1978. J. Mol. Biol.123, 149.
Ozawa, H. 1970. Biochemistry9, 2158.
Kopple, K.D., et al. 1962. J. Am. Chem. Soc.84, 4457.
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
표적 기관
Stomach,large intestine,lymph node
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.