324693
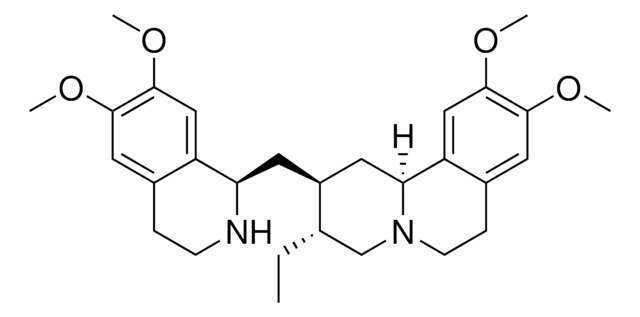
Emetine dihydrochloride
≥98% (HPLC), solid, protein synthesis blocker, Calbiochem®
동의어(들):
Emetine, Dihydrochloride, 6ʹ,7ʹ,10,11-Tetramethoxyemetan, 2HCl
About This Item
추천 제품
product name
Emetine, Dihydrochloride, Principal alkaloid of ipecac, isolated from the ground roots of Uragoga ipecacuanha.
Quality Level
분석
≥98% (HPLC)
형태
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
desiccated (hygroscopic)
protect from light
색상
white to off-white
white
solubility
water: 20 mg/mL
ethanol: soluble
배송 상태
ambient
저장 온도
2-8°C
InChI
1S/C29H40N2O4.2ClH/c1-6-18-17-31-10-8-20-14-27(33-3)29(35-5)16-23(20)25(31)12-21(18)11-24-22-15-28(34-4)26(32-2)13-19(22)7-9-30-24;;/h13-16,18,21,24-25,30H,6-12,17H2,1-5H3;2*1H/t18-,21-,24+,25-;;/m0../s1
InChI key
JROGBPMEKVAPEH-GXGBFOEMSA-N
일반 설명
생화학적/생리학적 작용
Movement of ribosomes along the mRNA
경고
재구성
기타 정보
Khan, M.A. 1995. Prog. Neurobiol.46, 541.
Kokuho, T., et al. 1995. Immunobiology193, 42.
Lee, Y.S., and Wurster, R.D. 1995. Cancer Lett.93, 157.
Burhans, W.C., et al. 1991. EMBO J. 10, 4351.
Filley, E.A., and Rook, G.A. 1991. Infect. Immun.59, 2567.
Landis, R.C., et al. 1991. J. Immunol.146, 128.
Schweighoffer, T., et al. 1991. Histochemistry96, 93.
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.