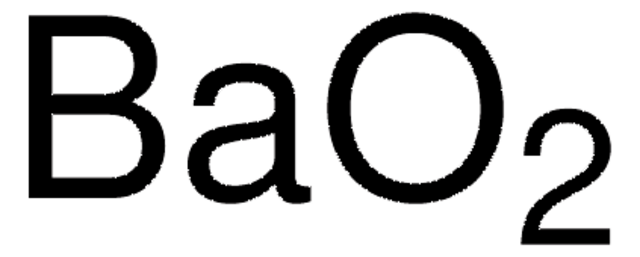추천 제품
일반 설명
Barium peroxide (BaO2) is commonly used as a strong oxidizing agent in chemical industries due to its high reactivity and high storage stability under inert conditions. It is also used in fireworks because it produces a bright green color when ignited.
애플리케이션
Barium peroxide can also be used in the synthesis of hexagonal barium ferrite magnets (BaFe12O19) by SHS reaction (self-propagating high-temperature synthesis) in the presence of Fe and Fe2O3. Barium ferrite magnetic components are the most commonly used in a wide range of products, from televisions and computer monitors to kitchen cabinet magnetic clasps.
BaO2 can also be used as a:
It can also find application in various other fields such as CO2 adsorption, disinfection, luminol-driven chemiluminescence, and wastewater treatment.
BaO2 can also be used as a:
- Bleaching agent in industrial bleach applications.
- Catalyst to start an aluminothermic reaction.
- Oxidizer in various pyrotechnic mixtures.
- Green flare in tracer ammunition.
It can also find application in various other fields such as CO2 adsorption, disinfection, luminol-driven chemiluminescence, and wastewater treatment.
법적 정보
Technipur is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1B
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Hydrogen Peroxide
Kirk-Othmer Encyclopedia of Chemical Technology (2001)
Binary and ternary pyrotechnic systems of Mn and/or Mo and BaO2 and/or SrO2: Part 2. Combustion studies
Drennan RL
Thermochimica Acta, 208, 223-246 (1992)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.