추천 제품
Grade
certified reference material
형태
liquid
특징
Snap-N-Spike®/Snap-N-Shoot®
포장
ampule of 1 mL
제조업체/상표
Cerilliant®
농도
100 μg/mL in acetonitrile
기술
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
응용 분야
clinical testing
형식
single component solution
저장 온도
−20°C
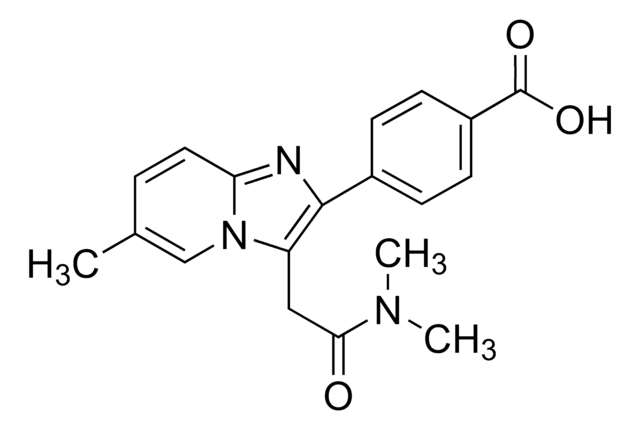
SMILES string
O=C1C2=NC=CN=C2C(OC(N3C([2H])([2H])CN(C)CC3([2H])[2H])=O)N1C4=NC=C(Cl)C=C4
InChI
1S/C17H17ClN6O3/c1-22-6-8-23(9-7-22)17(26)27-16-14-13(19-4-5-20-14)15(25)24(16)12-3-2-11(18)10-21-12/h2-5,10,16H,6-9H2,1H3/i8D2,9D2
InChI key
GBBSUAFBMRNDJC-LZMSFWOYSA-N
일반 설명
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
35.6 °F - closed cup
Flash Point (°C)
2 °C - closed cup
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.