857130P
Avanti
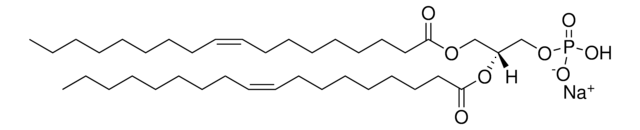
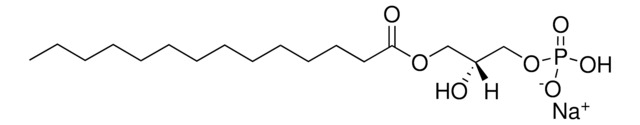
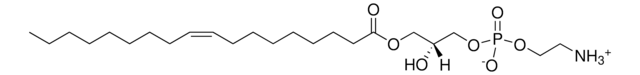
18:1 Lyso PA
Avanti Research™ - A Croda Brand
동의어(들):
oleoyl lysophosphatidic acid; 1-(9Z-octadecenoyl)-sn-glycero-3-phosphate (sodium salt); PA(18:1(9Z)/0:0); 18:1 LPA; o-LPA; 110681
About This Item
추천 제품
설명
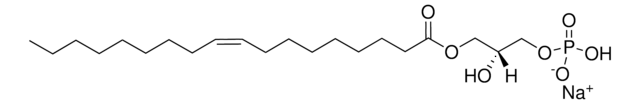
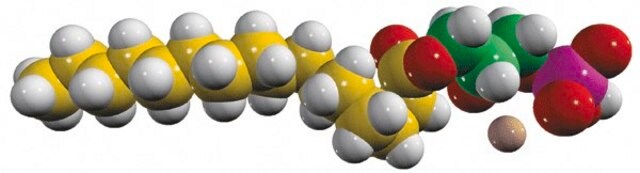
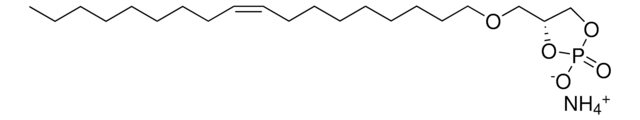
1-oleoyl-2-hydroxy-sn-glycero-3-phosphate (sodium salt)
분석
>99% (LPA; may contain up to 10% of the 2-LPA isomer, TLC)
양식
powder
포장
pkg of 1 × 1 g (857130P-1g)
pkg of 2 × 100 mg (857130P-200mg)
pkg of 1 × 25 mg (857130P-25mg)
제조업체/상표
Avanti Research™ - A Croda Brand
지질 유형
cardiolipins
phospholipids
배송 상태
dry ice
저장 온도
−20°C
SMILES string
O[C@](COP([O-])(O)=O)([H])COC(CCCCCCC/C=C\CCCCCCCC)=O.[Na+]
InChI
1S/C21H41O7P.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(23)27-18-20(22)19-28-29(24,25)26;/h9-10,20,22H,2-8,11-19H2,1H3,(H2,24,25,26);/q;+1/p-1/b10-9-;/t20-;/m1./s1
InChI key
XGRLSUFHELJJAB-JGSYTFBMSA-M
일반 설명
애플리케이션
생화학적/생리학적 작용
포장
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.