추천 제품
Quality Level
분석
≥99%
bp
220 °C (lit.)
mp
30-32 °C (lit.)
density
1.049 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
InChI key
CUONGYYJJVDODC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Malononitrile, a weak cyanocarbon acid, is a versatile compound with exceptional reactivity. This crystalline aliphatic nitrile is used as a building block to synthesize heterocyclic compounds and polymers
애플리케이션
Malononitrile may be used in the:
- base-promoted on-water synthesis of [1,6]-naphthyridines.†
- synthesis of γ-ketoamides.
- preparation of heterocyclic privileged medicinal scaffolds involving pyridine, 1,4-dihydropyridine, chromeno[2,3-b]pyridine and dihydro-1,4-dithiepine frameworks.
포장
Packaged in glass bottles
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
186.8 °F - closed cup
Flash Point (°C)
86 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
The chemistry of malononitrile.
F Freeman
Chemical reviews, 69(5), 591-624 (1969-10-01)
Enxiang Wei et al.
Organic & biomolecular chemistry, 12(33), 6389-6392 (2014-07-11)
An efficient synthesis of γ-ketoamides was developed by the one-pot multicomponent reaction of chalcones, malononitrile and DMF (as both the reactant and solvent) in the presence of NaOH (3.0 equiv.). The reaction features high atom economy, easily available starting materials
Nikolai M Evdokimov et al.
The Journal of organic chemistry, 72(9), 3443-3453 (2007-04-06)
Heterocyclic privileged medicinal scaffolds involving pyridine, 1,4-dihydropyridine, chromeno[2,3-b]pyridine, and dihydro-1,4-dithiepine frameworks are prepared via a single-step multicomponent reaction of structurally diverse aldehydes with various thiols and malononitrile. Mechanistic studies of the synthetic pathway leading to pyridines reveal that 1,4-dihydropyridines undergo
Kamila K Mentel et al.
Nature communications, 9(1), 2903-2903 (2018-07-27)
Electron transfer reactions are arguably the simplest chemical reactions but they have not yet ceased to intrigue chemists. Charge-separation and charge-recombination reactions are at the core of life-sustaining processes, molecular electronics and solar cells. Intramolecular electron donor-acceptor systems capture the
Ananta Kumar Atta et al.
Organic letters, 15(5), 1072-1075 (2013-02-21)
The Hg(II)-specific intramolecular cyclization reaction of ethynyl phenols was carried out for the first time in semiaqueous media at ambient temperature. The reaction unit (ethynyl phenol) was coupled with a malononitrile derivative (signal unit), which afforded the chromogenic Hg(II) indicator
문서
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
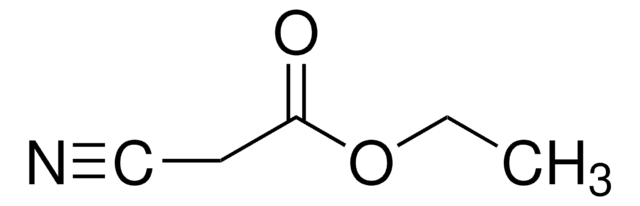
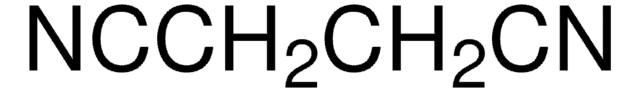






![2-[Bis(methylthio)methylene]malononitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)