추천 제품
분석
98%
mp
202-206 °C (lit.)
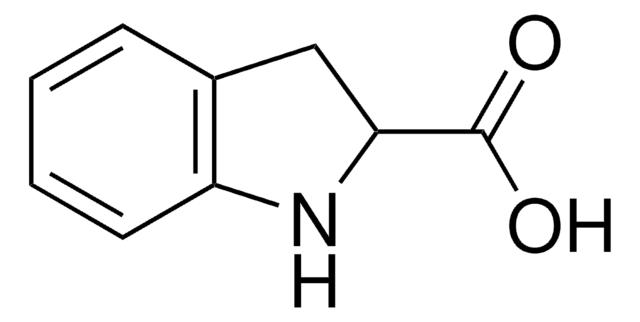
SMILES string
OC(=O)c1cc2ccccc2[nH]1
InChI
1S/C9H7NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-5,10H,(H,11,12)
InChI key
HCUARRIEZVDMPT-UHFFFAOYSA-N
유전자 정보
human ... SRD5A1(6715)
rat ... Grin2a(24409)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
- Reactant for total synthesis of (±)-dibromophakellin and analogs
- Reactant for synthesis of the pyrrolizidine alkaloid (±)-trachelanthamidine
- Reactant for stereoselective preparation of renieramycin G analogs
- Reactant for preparation of spirooxoindolepyrrolidines via reduction of indole-2-carboxylic acid followed by oxidation, condensation, reduction, amidation and Kharasch radical cyclization
- Reactant for Pd-catalyzed cyclization
- Reactant for preparation of N,N′-(pentane)diylbis[indolecarboxamide] and N,N′-[phenylenebis(methylene)]bis[indolecarboxamide] derivatives
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
T Tonohiro et al.
General pharmacology, 28(4), 555-560 (1997-04-01)
1. A putative agonist for the strychnine-sensitive glycine receptor picolinic acid was tested for its anticonvulsant activities in mice and muscle-relaxant activities in rats and compared with indole-2-carboxylic acid (I2CA), an antagonist for the strychnine-insensitive glycine receptor. Their effects on
James F Dropinski et al.
Bioorganic & medicinal chemistry letters, 15(22), 5035-5038 (2005-09-13)
A series of novel aryl indole-2-carboxylic acids has been identified as potent selective PPARgamma modulators. Their chemical synthesis and in vitro activities are discussed. Compound 5 was selected for in vivo testing in the db/db mouse model of type 2
R Rama Suresh et al.
The Journal of organic chemistry, 77(16), 6959-6969 (2012-07-26)
Two methodologies, one involving Ar-I reactivity and the other through C-H functionalization, for the formation of indolo[2,3-c]pyrane-1-ones via the corresponding allenes, are presented. A highly efficient approach to indolo[2,3-c]pyrane-1-one derivatives through the Pd-catalyzed regioselective annulation of allenes with 3-iodo-1-alkylindole-2-carboxylic acids
Hideyuki Shiozawa et al.
Journal of the American Chemical Society, 124(15), 3914-3919 (2002-04-11)
Glycopeptide antibiotics of the vancomycin group bind to bacterial cell wall analogue precursors, and typically also form dimers. We have studied the interplay between these two sets of noncovalent bonds formed at separate interfaces. Indole-2-carboxylic acid (L) forms a set
Gopinadhan N Anilkumar et al.
Bioorganic & medicinal chemistry letters, 21(18), 5336-5341 (2011-08-16)
SAR development of indole-based palm site inhibitors of HCV NS5B polymerase exemplified by initial indole lead 1 (NS5B IC(50)=0.9 μM, replicon EC(50)>100 μM) is described. Structure-based drug design led to the incorporation of novel heterocyclic moieties at the indole C3-position
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.