추천 제품
제품명
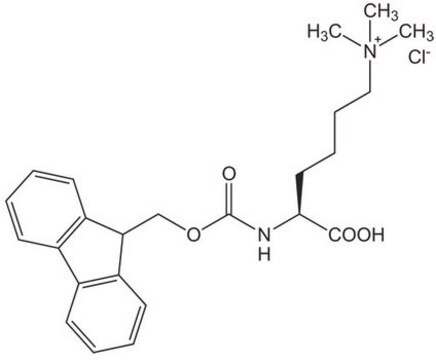
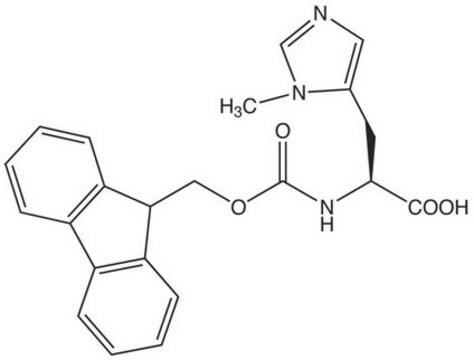
Fmoc-Lys(Me)3-OH Chloride, ≥97%
Quality Level
분석
≥97%
양식
liquid
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
응용 분야
peptide synthesis
작용기
Fmoc
배송 상태
dry ice
저장 온도
−20°C
InChI
1S/C24H30N2O4.ClH/c1-26(2,3)15-9-8-14-22(23(27)28)25-24(29)30-16-21-19-12-6-4-10-17(19)18-11-5-7-13-20(18)21;/h4-7,10-13,21-22H,8-9,14-16H2,1-3H3,(H-,25,27,28,29);1H/t22-;/m0./s1
InChI key
XUJRNPVABVHOAJ-FTBISJDPSA-N
일반 설명
Fmoc protected N-trimethyl lysine
애플리케이션
Fmoc-Lys(Me)3-OH chloride is one of the common N-terminal protected reagents used in the peptide synthesis. Some of the reported examples are:
- Synthesis of peptide linkers for monoclonal antibody-auristatin F conjugates.
- Preparation of multifunctional reagents with enhanced ionization properties for the analysis of protein modification in human cells and dynamic profiling of protein lipidation.
- Sequential peptide ligation to synthesize histone H3 containing a trimethyl lysine residue, with modified tail region.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Multifunctional Reagents for Quantitative Proteome?Wide Analysis of Protein Modification in Human Cells and Dynamic Profiling of Protein Lipidation During Vertebrate Development.
Broncel M, et al.
Angewandte Chemie (International Edition in English), 54(20), 5948-5951 (2015)
Novel peptide linkers for highly potent antibody? auristatin conjugate.
Doronina S O, et al.
Bioconjugate Chemistry, 19(10), 1960-1963 (2008)
Sequential Peptide Ligation by Combining the Cys?Pro Ester (CPE) and Thioester Methods and Its Application to the Synthesis of Histone H3 Containing a Trimethyl Lysine Residue.
Kawakami T, et al.
Bulletin of the Chemical Society of Japan, 86(6), 690-697 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.