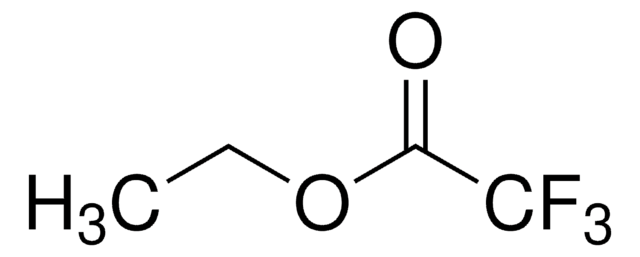
추천 제품
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.375 (lit.)
bp
129-130 °C (lit.)
density
1.259 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)CC(=O)C(F)(F)F
InChI
1S/C6H7F3O3/c1-2-12-5(11)3-4(10)6(7,8)9/h2-3H2,1H3
InChI key
OCJKUQIPRNZDTK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Ethyl 4,4,4-trifluoroacetoacetate (ETFAA) is a general reagent to synthesize enantiopure trifluoromethyl-functionalized products. Applications include:
- Synthesis of (S)- and (R)-α-trifluoromethyl-aspartic acid and α- trifluoromethyl-serine from chiral CF3-oxazolidines, which is derived from ETFAA.
- Enantiopure synthesis of trifluoromethyl-β-amino acid derivatives.
- Synthesis of (2R)-2-trifluoromethyl-2-carboxyazetidine, (R)- and (S)-trifluoromethylhomoserines from oxazolidine intermediate obtained by condensing (R)-phenylglycinol with ETFAA.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
100.4 °F - closed cup
Flash Point (°C)
38 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Straightforward Synthesis of Novel Enantiopure α-Trifluoromethylated Azetidine 2-Carboxylic Acid and Homoserines.
Lensen N, et al.
Organic Letters, 17(2), 342-345 (2015)
Concise synthesis of enantiopure (S)-and (R)-α-trifluoromethyl aspartic acid and α-trifluoromethyl serine from chiral trifluoromethyl oxazolidines (Fox) via a Strecker-type reaction.
Simon J, et al.
Tetrahedron Asymmetry, 22(3), 309-314 (2011)
Ethyl-4, 4, 4-trifluoroacetoacetate (ETFAA), a powerful building block for enantiopure chirons in trifluoromethyl-β-amino acid series.
Michaut V, et al.
Journal of Fluorine Chemistry, 128(8), 889-895 (2007)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.