D214256
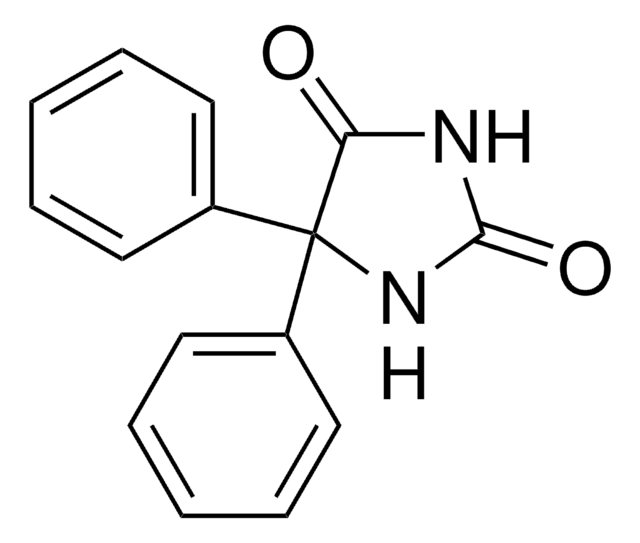
5,5-Diphenyl-2-thiohydantoin
99%
동의어(들):
5,5-Diphenyl-2-thioxo-4-imidazolidinone, 5,5-Diphenylimidazolidine-4-one-2-thione, DPTH
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C15H12N2OS
CAS Number:
Molecular Weight:
268.33
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
양식:
powder
분석:
99%
추천 제품
분석
99%
양식
powder
mp
237-239 °C (lit.)
SMILES string
O=C1NC(=S)NC1(c2ccccc2)c3ccccc3
InChI
1S/C15H12N2OS/c18-13-15(17-14(19)16-13,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H2,16,17,18,19)
InChI key
AMDPNECWKZZEBQ-UHFFFAOYSA-N
유전자 정보
human ... CNR1(1268) , CNR2(1269)
rat ... Faah(29347)
애플리케이션
Reactant for synthesis of:
- Imidazole derivatives
- Imidazothiazole and glycocyamidine derivatives for antimicrobial studies
- Fatty acid amide hydrolase inhibitor templates
- Anti-cancer agents
- Hydantoins and thiohydantoins
- Acyl CoA: cholesterol acyltransferase inhibitors
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
H Gerber et al.
Endocrinology, 135(6), 2688-2699 (1994-12-01)
Some years ago, we reported that colloid goiters could be produced experimentally in mice and rats by injection of TSH over a few days in the presence of ample iodine supply. This clearly showed that colloid accumulation and intense TSH
Yuan Liu et al.
Cancer letters, 271(2), 294-305 (2008-07-25)
Previously, we demonstrated that 5,5-diphenyl-2-thiohydantoin (DPTH) exerts an anti-proliferation effect on subcultured human umbilical vein endothelial cells (HUVEC). In the present study, we show that 2(naphthalen-2-ylmethylsulfanyl)-5,5-diphenyl-1,5-dihydro-imidazol-4-one (DPTH-N10), a derivative compound of DPTH, exerts a 5 times stronger inhibition of [3H]thymidine
Hitoshi Yoshino et al.
Bioorganic & medicinal chemistry, 18(23), 8150-8157 (2010-11-06)
A series of 5,5-dimethylthiohydantoin derivatives were synthesized and evaluated for androgen receptor pure antagonistic activities for the treatment of castration-resistant prostate cancer. Since CH4933468, which we reported previously, had a problem with agonist metabolites, novel thiohydantoin derivatives were identified by
Cheng-Kuo Cheng et al.
Vascular pharmacology, 48(2-3), 138-142 (2008-02-26)
Previously, we identified DPTH, an analogue of antiepileptic drug phenytoin (5,5-diphenylhydantoin, DPT), capable of retarding the cell cycle in the human vascular endothelial cells. Our data suggest that DPTH inhibits human umbilical venous endothelial cells (HUVEC) proliferation by increasing the
Naimeh Moshtael Arani et al.
Ultrasonics sonochemistry, 18(2), 640-643 (2010-10-06)
To obtain a rapid, efficient and mild synthesis of 5,5-diphenylhydantoin and 5,5-diphenyl-2-thiohydantoin derivatives, ultrasonic irradiation has been applied to the reaction mixtures containing substituted benzils and urea or thiourea derivatives catalyzed by KOH in DMSO/H(2)O, which allowed us to achieve
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.