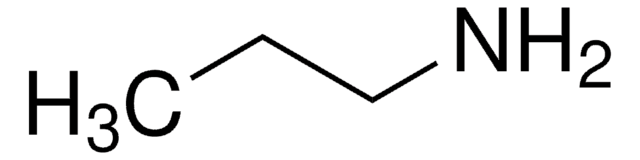
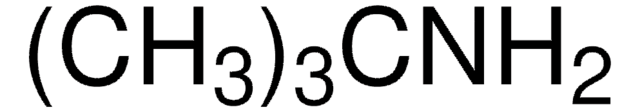추천 제품
일반 설명
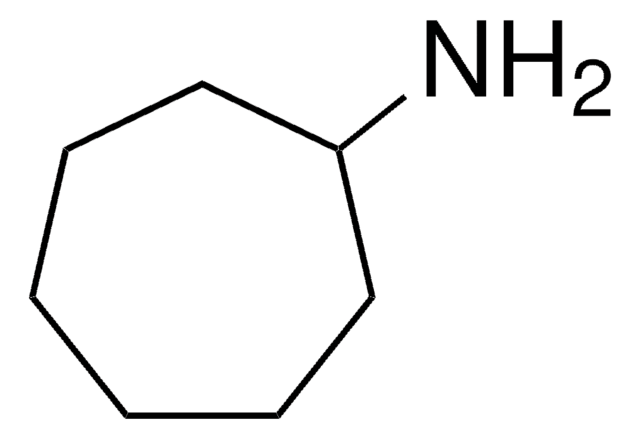
Cycloheptylamineis a versatile compound that has several useful applications in organicsynthesis. Its ability to act as a building block, catalyst, and reagent makesit a valuable tool for the development of new organic compounds. It is alsoused as a reagent for the functionalization of organic molecules.
애플리케이션
Cycloheptylamine is a building block for the formation of an ABX3-typed perovskite structure compound [C5H9–NH3][CdCl3].
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
52.7 °F - closed cup
Flash Point (°C)
11.5 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
D G Craciunescu et al.
In vivo (Athens, Greece), 1(4), 229-234 (1987-07-01)
Ten new Pt (II) complexes were synthesized and tested as potential antitumor drugs in vitro on KB human tumour cell line, and in vivo against four experimental tumour systems (P388, L1210, ADJ/PC6A and Yoshida sarcoma). The complexes contained two primary
M J Comin et al.
Nucleosides & nucleotides, 18(10), 2219-2231 (2000-01-05)
Purine carbanucleosides built on a 6-oxabicyclo[3.1.0]hexane template were synthesized from readily available 2-cyclopentenone employing a Mitsunobu reaction to incorporate the base onto the carbocyclic ring. Both adenosine and guanosine analogues exhibited moderate antiviral activity.
Maris Vilums et al.
Journal of medicinal chemistry, 56(19), 7706-7714 (2013-09-14)
Preclinical models of inflammatory diseases (e.g., neuropathic pain, rheumatoid arthritis, and multiple sclerosis) have pointed to a critical role of the chemokine receptor 2 (CCR2) and chemokine ligand 2 (CCL2). However, one of the biggest problems of high-affinity inhibitors of
M S Sansom et al.
Protein engineering, 6(1), 65-74 (1993-01-01)
The influenza A M2 protein forms cation-selective ion channels which are blocked by the anti-influenza drug amantadine. A molecular model of the M2 channel is presented in which a bundle of four parallel M2 transbilayer helices surrounds a central ion-permeable
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.