추천 제품
vapor pressure
4.67 psi ( 20 °C)
Quality Level
분석
98%
양식
liquid
autoignition temp.
527 °F
refractive index
n20/D 1.420 (lit.)
bp
49-50 °C (lit.)
density
0.824 g/mL at 25 °C (lit.)
SMILES string
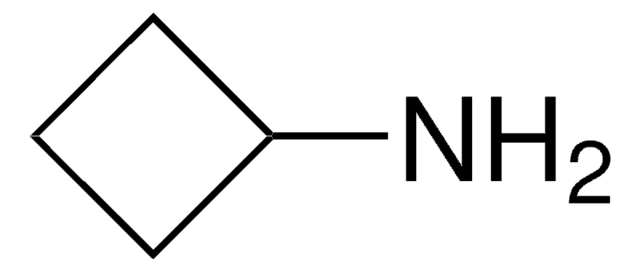
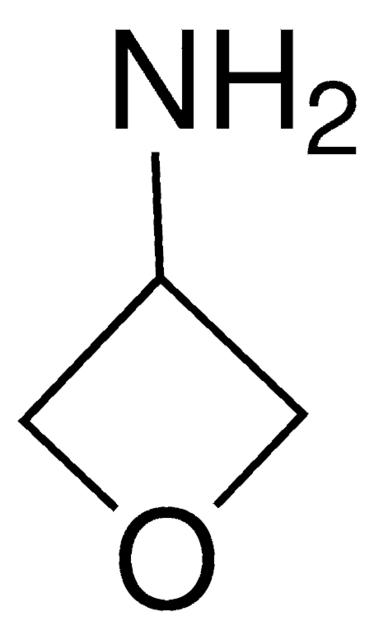
NC1CC1
InChI
1S/C3H7N/c4-3-1-2-3/h3H,1-2,4H2
InChI key
HTJDQJBWANPRPF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Cyclopropylamine(CPA) has been used in the synthesis of N-[4-(4-fluoro)phenyl-2-aminothiazol-5-yl]pyrimidin-2-yl-alkylamine derivatives. It has been used in the synthesis of Pt(CPA)2(bismethylthiomethylenepropanedioate) and Pt(CPA)2(bisethylthiomethylenepropanedioate) complexes.
생화학적/생리학적 작용
Cyclopropylamine inactivates cytochrome P450 enzymes by a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme. It is a mechanism-based inhibitor of quinoprotein methylamine dehydrogenase from Paracoccus denitrificans.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
33.8 °F - closed cup
Flash Point (°C)
1 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
Synthesis and Antifungal Activity of 5-[2-(Alkylamino) pyrimidin-4-yl]-4-phenylthiazol-2-cycloalkylamine Derivatives on Phytophthora capsici.
Nam Sw, et al.
J. Korean Chem. Soc., 54(3), 395-402 (2011)
Coordination Mode vs. Anticancer Activity of the Platinum (II) Complexes Involving Sulfur-Containing Ylidenemalonate Ligands.
Sakai N, et al.
Bull. Korean Chem. Soc., 19(12), 1377-1379 (1998)
Dapeng Sun et al.
FEBS letters, 517(1-3), 172-174 (2002-06-14)
Cyclopropylamine is a mechanism-based inhibitor of the quinoprotein methylamine dehydrogenase (MADH) from Paracoccus denitrificans. The resulting inactivation is accompanied by the formation of a covalent cross-link between the alpha and beta subunits of MADH. The results of site-directed mutagenesis studies
Bram Denolf et al.
Organic letters, 9(2), 187-190 (2007-01-16)
Treatment of novel chiral N-sulfinyl alpha-chloro ketimines with Grignard reagents resulted in the synthesis of chiral N-(1-substituted cyclopropyl)-tert-butanesulfinamides in acceptable to good yields and diastereoselectivity via 1,3-dehydrohalogenation and subsequent addition of the Grignard reagent to the intermediate cyclopropylideneamine. Only in
Soda Chanthamath et al.
Organic letters, 15(4), 772-775 (2013-01-31)
The Ru(II)-Pheox-catalyzed asymmetric cyclopropanation of vinylcarbamates with diazoesters resulted in the corresponding cyclopropylamine derivatives in high yield and excellent diastereoselectivity (up to 96:4) and enantioselectivity (up to 99% ee).
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 125504-25G | 4061838723628 |
| 125504-10G | 4061838723611 |
| 125504-10KG |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.