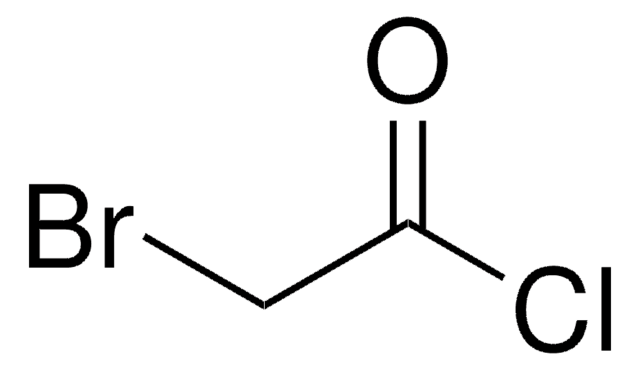
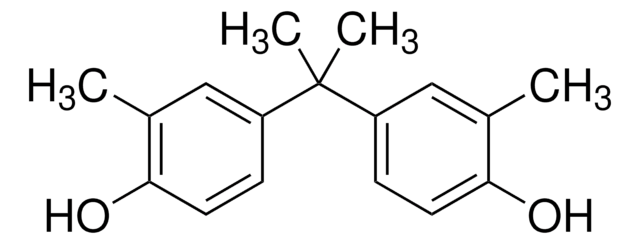
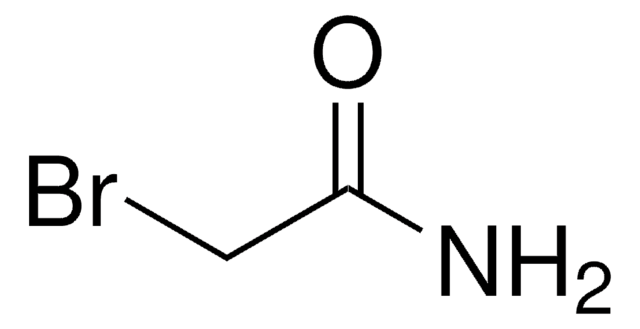
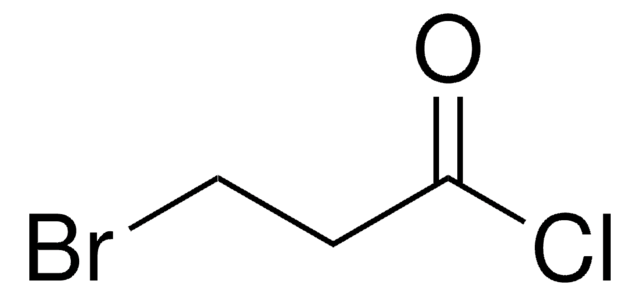
추천 제품
vapor pressure
3.8 mmHg ( 25 °C)
Quality Level
분석
≥98%
양식
liquid
refractive index
n20/D 1.547 (lit.)
bp
147-150 °C (lit.)
density
2.317 g/mL at 25 °C (lit.)
저장 온도
2-8°C
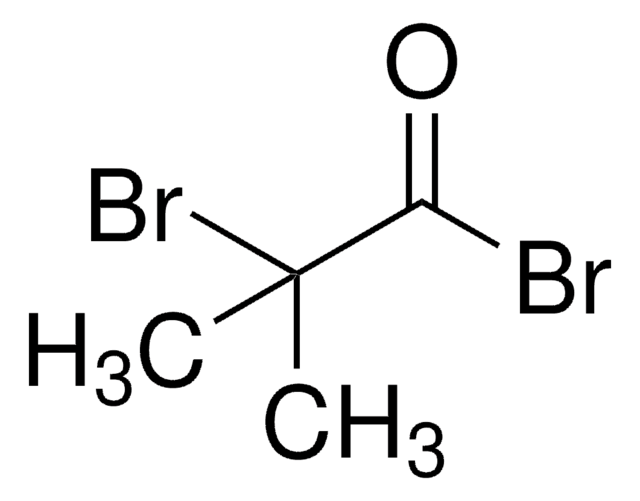
SMILES string
BrCC(Br)=O
InChI
1S/C2H2Br2O/c3-1-2(4)5/h1H2
InChI key
LSTRKXWIZZZYAS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Bromoacetyl bromide is employed as an acylating reagent and in the production of heterocyclic compounds.
애플리케이션
Bromoacetyl bromide can be used to convert:
It can also be used as a reagent to synthesize imidazolium or piridinium based ionic liquids.
- Amines to azido acetamides.
- p-Arsanilic acid to 4-(2-bromoacetylamino)benzenearsonic acid, a precursor to 4-(N-(S-penicillaminylacetyl)amino)phenylarsonous acid (PENAO), a metal-based drug.
- 3,5-Dimethylphenol to 3,5-dimethylphenyl 2-bromoacetate.
- Cystamine dihydrochloride to N,N′-bis(bromoacetyl) cystamine, a bifunctional quaternizing agent.
It can also be used as a reagent to synthesize imidazolium or piridinium based ionic liquids.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
보충제 위험성
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
221.0 °F - closed cup
Flash Point (°C)
105 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Aggregation behavior and antimicrobial activity of ester-functionalized imidazolium-and pyridinium-based ionic liquids in aqueous solution.
Garcia MT, et al.
Langmuir, 29(8), 2536-2545 (2013)
pH and reduction dual-responsive nanogel cross-linked by quaternization reaction for enhanced cellular internalization and intracellular drug delivery.
Li M, et al.
Polym. Chem., 4(4), 1199-1207 (2013)
Direct Polymerization of the Arsenic Drug PENAO to Obtain Nanoparticles with High Thiol-Reactivity and Anti-Cancer Efficiency.
Noy JM, et al.
Bioconjugate Chemistry, 29(2), 546-558 (2018)
Regio-and Stereoselective Nickel-Catalyzed Coupling of Boronic Acids with Allenoates.
Liu Y, et al.
Synthesis, 29(2), 546-558 (2018)
Site-Selective Conversion of Azido Groups at Carbonyl ?-Positions to Diazo Groups in Diazido and Triazido Compounds.
Yokoi T, et al.
The Journal of Organic Chemistry, 83(19), 12103-12121 (2018)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.