A2898
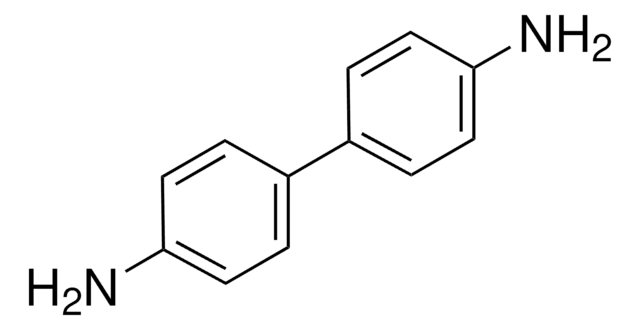
4-Aminobiphenyl
≥98%
동의어(들):
4-Aminodiphenyl, 4-Biphenylamine, 4-Biphenylylamine, 4-Phenylaniline, Biphenyl-4-ylamine, NSC 7660, Xenylamine
About This Item
추천 제품
분석
≥98%
양식
solid
autoignition temp.
842 °F
bp
191 °C/15 mmHg (lit.)
mp
52-54 °C (lit.)
SMILES string
Nc1ccc(cc1)-c2ccccc2
InChI
1S/C12H11N/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9H,13H2
InChI key
DMVOXQPQNTYEKQ-UHFFFAOYSA-N
유전자 정보
human ... UGT1A4(54657)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Induces DNA damage (carcinogen) in human bladder carcinoma cells and bladder tissue in mouse
- Synthetic amine ligand for enrichment, depletion and one-step purification of leech proteins
생화학적/생리학적 작용
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1A
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
프로토콜
US EPA Method 8270 (Appendix IX): GC Analysis of Semivolatiles on Equity®-5 (30 m x 0.25 mm I.D., 0.50 μm)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.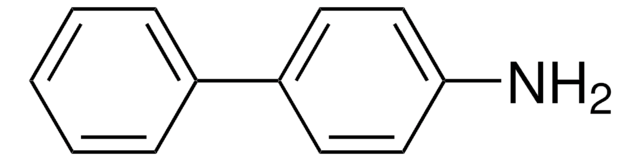

![Benzo[a]pyrene ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)

