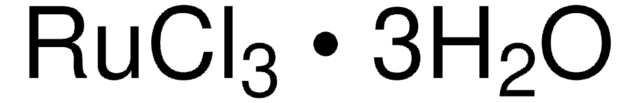931578
Ruthenium(III) chloride hydrate
≥99.9% trace metals basis
동의어(들):
Ruthenium trichloride, Trichlororuthenium hydrate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
RuCl3 · xH2O
CAS Number:
Molecular Weight:
207.43 (anhydrous basis)
MDL number:
UNSPSC 코드:
12352302
NACRES:
NA.21
분석:
≥99.9% trace metals basis
양식:
powder
solubility:
acetone: soluble ((lit.))
ethanol: soluble
water: soluble
ethanol: soluble
water: soluble
추천 제품
Quality Level
분석
≥99.9% trace metals basis
양식
powder
불순물
≤1000.0 ppm (trace metals analysis)
solubility
acetone: soluble ((lit.))
ethanol: soluble
water: soluble
density
3.11 g/cm3
SMILES string
O.Cl[Ru](Cl)Cl
InChI
1S/3ClH.H2O.Ru/h3*1H;1H2;/q;;;;+3/p-3
InChI key
BIXNGBXQRRXPLM-UHFFFAOYSA-K
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Ruthenium chloride hydrate is a dark brown or black solid often used as a powder. The hydrate is hygroscopic and is soluble in water, ethanol, acetone, and a wide range of polar organic solvents. The anhydrous form is insoluble.
Industrially, ruthenium trichloride hydrate is produced by dissolving ruthenium oxides in hydrochloric acid. The hydrated salt is obtained by recrystallization.
Industrially, ruthenium trichloride hydrate is produced by dissolving ruthenium oxides in hydrochloric acid. The hydrated salt is obtained by recrystallization.
애플리케이션
Ruthenium chloride is most used as a precursor for the synthesis of ruthenium complexes. Our RuCl3·H2O with 99.9% trace metals purity is well-suited for applications in materials science. One common application of ruthenium trichloride hydrate is in the synthesis of ruthenium nanoparticles, which are used as catalysts or composited with other materials and used as co-catalysts for both oxygen and hydrogen evolution reactions. Researchers have used our ruthenium chloride hydrate to produce high-quality, catalytically active ruthenium nanoparticles and ruthenium oxide nanoparticles.
Another common application of ruthenium chloride hydrate is as a precursor for single-atom catalysts. For example, scientists have used ruthenium chloride hydrate for the synthesis of ruthenium single-atom-doped ZrO2 particles to catalyze nitrogen fixation and for the synthesis of ruthenium single-atom-doped MXenes to catalyze hydrogen evolution. A third common application of ruthenium chloride hydrate is in the synthesis of metal alloys, like PtRuIr, or PtRuFe, which are investigated for electrocatalysis, usually the oxidation of simple organics like methanol or formic acid.
For use in all these applications, also consider our higher-purity ruthenium chloride hydrate, 463779, with trace metals purity greater than 99.98%, which offers the best reproducibility and purity.
Another common application of ruthenium chloride hydrate is as a precursor for single-atom catalysts. For example, scientists have used ruthenium chloride hydrate for the synthesis of ruthenium single-atom-doped ZrO2 particles to catalyze nitrogen fixation and for the synthesis of ruthenium single-atom-doped MXenes to catalyze hydrogen evolution. A third common application of ruthenium chloride hydrate is in the synthesis of metal alloys, like PtRuIr, or PtRuFe, which are investigated for electrocatalysis, usually the oxidation of simple organics like methanol or formic acid.
For use in all these applications, also consider our higher-purity ruthenium chloride hydrate, 463779, with trace metals purity greater than 99.98%, which offers the best reproducibility and purity.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions
Lee, Y., Suntivich, J., et al.
The Journal of Physical Chemistry Letters, 3, 399?404-399?404 (2012)
Vinoth Ramalingam et al.
Advanced materials (Deerfield Beach, Fla.), 31(48), e1903841-e1903841 (2019-10-18)
A titanium carbide (Ti3 C2 Tx ) MXene is employed as an efficient solid support to host a nitrogen (N) and sulfur (S) coordinated ruthenium single atom (RuSA ) catalyst, which displays superior activity toward the hydrogen evolution reaction (HER).
Javeed Mahmood et al.
Nature nanotechnology, 12(5), 441-446 (2017-02-14)
The hydrogen evolution reaction (HER) is a crucial step in electrochemical water splitting and demands an efficient, durable and cheap catalyst if it is to succeed in real applications. For an energy-efficient HER, a catalyst must be able to trigger
Fabing Su et al.
Journal of the American Chemical Society, 129(46), 14213-14223 (2007-11-02)
We report here a thermal reduction method for preparing Ru catalysts supported on a carbon substrate. Mesoporous SBA-15 silica, surface-carbon-coated SBA-15, templated mesoporous carbon, activated carbon, and carbon black with different pore structures and compositions were employed as catalyst supports
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.