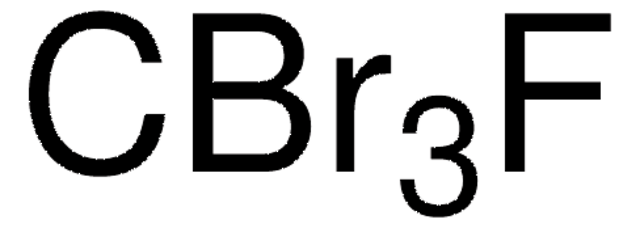추천 제품
분석
≥95.0%
형태
solid
저장 온도
2-8°C
InChI
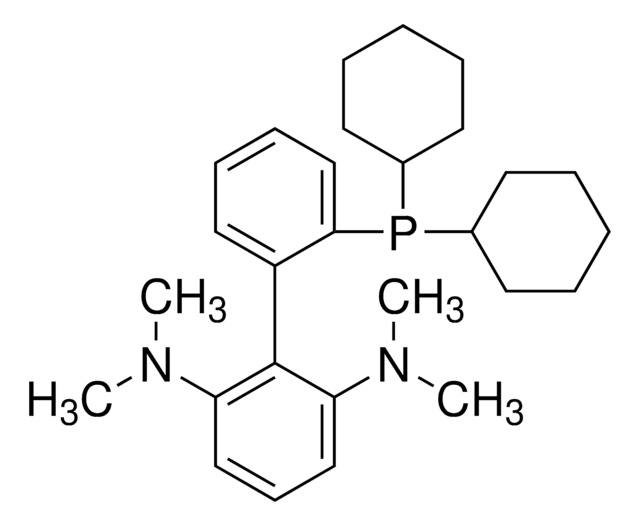
1S/C19H16F2P.BrH/c20-19(21)22(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18;/h1-15,19H;1H/q+1;/p-1
InChI key
WNPMJTVOWUTTSY-UHFFFAOYSA-M
애플리케이션
When irradiated with visible light, difluoromethyltriphenylphosphonium bromide generates a difluoromethyl radical, which has been shown to react with alkenes, enamides, and thiols to give the difluoromethylated product.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Qing-Yu Lin et al.
Angewandte Chemie (International ed. in English), 55(4), 1479-1483 (2015-12-17)
Bromodifluoromethylphosphonium bromide was solely used as the precursor of difluorocarbene. Herein, an unprecedented visible-light-induced hydrodifluoromethylation of alkenes with bromodifluoromethylphosphonium bromide using H2O and THF as hydrogen sources for the synthesis of difluoromethylated alkanes is described. This difluoromethylation is characterized by
Niklas B Heine et al.
Organic letters, 19(15), 4150-4153 (2017-07-21)
A method for facile difluoromethylation of various thiols using (difluoromethyl)triphenylphosphonium bromide under mild reaction conditions is presented. The transformation proceeds in the absence of any transition metal using a bench-stable and readily accessible phosphonium salt. Deuterium labeling experiments and cyclic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.