792373
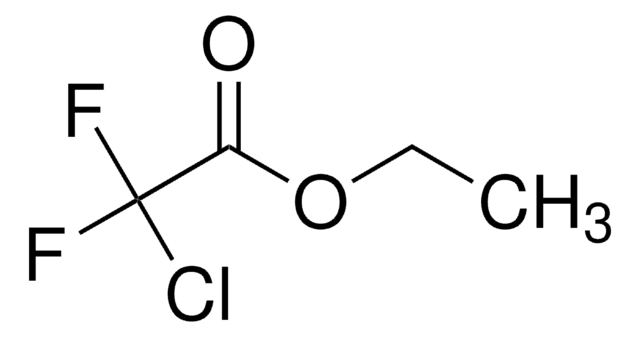
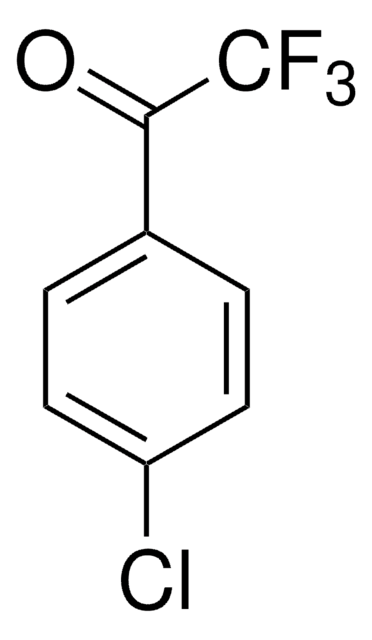
2-Chloro-2,2-difluoroacetophenone
95%
동의어(들):
2-Chloro-2,2-difluoro-1-phenylethanone, Chlorodifluoromethyl phenyl ketone, α-Chloro-α,α-difluoroacetophenone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5COCF2Cl
CAS Number:
Molecular Weight:
190.57
Beilstein:
2251448
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
형태
liquid
반응 적합성
reaction type: C-C Bond Formation
refractive index
n20/D 1.4954
n20/D 1.497 (lit.)
bp
94-96 °C/35 mmHg (lit.)
density
1.293 g/mL at 25 °C (lit.)
1.3178 g/mL at 25 °C
작용기
chloro
fluoro
ketone
phenyl
SMILES string
FC(F)(Cl)C(=O)c1ccccc1
InChI
1S/C8H5ClF2O/c9-8(10,11)7(12)6-4-2-1-3-5-6/h1-5H
InChI key
MNOONJNILVDLSW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Chloro-2,2-difluoroacetophenone is a difluorocarbene reagent, which is generally used in the synthesis of 2,2-difluoro enol silyl ethers, and gem -difluoromethene derived compounds.
애플리케이션
2-Chloro-2,2-difluoroacetophenone can be used:
- As a reagent in the difluoromethylation of various phenols to yield aryl difluoromethyl ethers.
- As a precursor in the Baylis-Hillman reaction of fluoroalkyl ketones to obtain chlorodifluoromethyl containing products.
- As a substrate in the synthesis of propargyl alcohols using a novel ruthenium catalyst.
Reagent is an effective product for the synthesis of difluoromethylated phenols in the presence of mild base and aqueous solvent.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
160.0 °F
Flash Point (°C)
71.1 °C
가장 최신 버전 중 하나를 선택하세요:
Laijun Zhang et al.
The Journal of organic chemistry, 71(26), 9845-9848 (2006-12-16)
A novel and non-ODS-based (ODS = ozone-depleting substance) preparation of 2-chloro-2,2-difluoroacetophenone (1) was achieved in high yield by using 2,2,2-trifluoroacetophenone as the starting material. Compound 1 was found to act as a good difluorocarbene reagent, which readily reacts with a
Octahedral ruthenium complex with exclusive metal-centered chirality for highly effective asymmetric catalysis
Zheng Y, et al.
Journal of the American Chemical Society, 139(12), 4322-4325 (2017)
2-Chloro-2, 2-difluoroacetophenone: a non-ODS-based difluorocarbene precursor and its use in the difluoromethylation of phenol derivatives
Zhang L, et al.
The Journal of Organic Chemistry, 71(26), 9845-9848 (2006)
Study of Fluorocarbonyls for the Baylis- Hillman Reaction
Ram Reddy MV, et al.
The Journal of Organic Chemistry, 67(15), 5382-5385 (2002)
2-Chloro-2,2-difluoroacetophenone
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2009)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.